Label: JACK BLACK INK BOOST TATTOO CARE- octinoxate, octisalate, zinc oxide lotion
- NDC Code(s): 66738-381-40
- Packager: Jack Black L.L.C
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
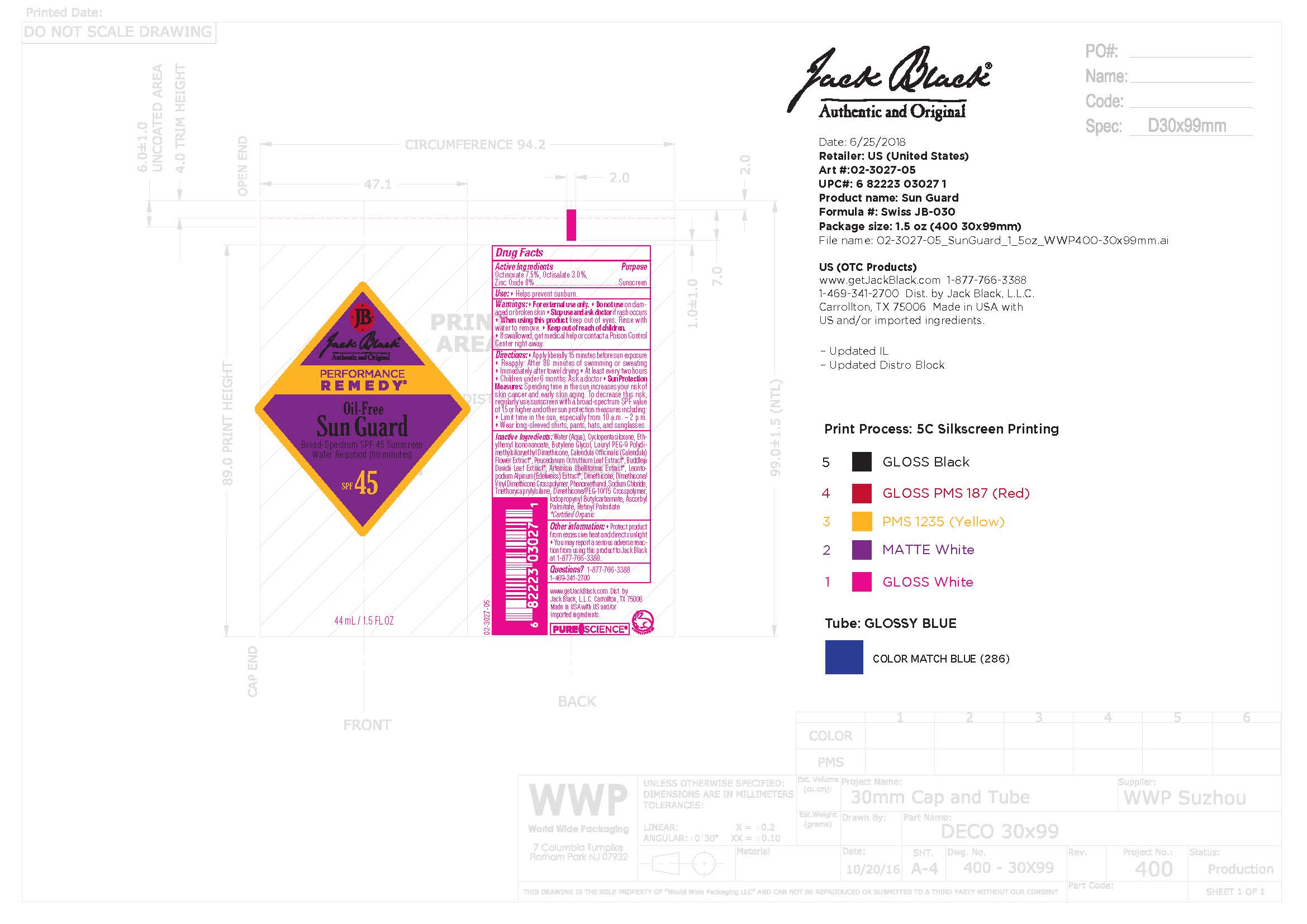
- Active Ingredient
- Purpose
- Uses
- Warnings
- DO NOT USE
- STOP USE
- WHEN USING
-
Directions
- Apply liberally15 minutes before sun exporsure
- Reapply: After 80 minutes of swimming or sweating
- Immediately after towel drying
- At least every two hours
- Children under 6 months: Ask a doctor
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use sunscreen with a broad-spectrum SPF value of 15 or higher and othe sun protection measures including:
- Limit time in the sun, expecially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- KEEP OUT OF REACH OF CHILDREN
-
Inactive Ingredients
Water (Aqua), Cyclopentasiloxane, Ethylhexyl Isononanoate, Butylene Glycol, Lauryl PEG-9 Polydimethylsiloxyethyl Dimethicone, Calendula Officinalis (Calendula) Flower Extract*, Peucedanum Ostruthium Leaf Extract*, Buddleja Davidii Leaf Extract*, Artemisia Ubelliformis Extract*, Leontopodium Alpinum (Edelweiss) Extract*, Dimethicone, Dimethicone/Vinyl Dimethicone Crosspolymer, Phenoxyethanol, Sodium Chloride, Triethoxycaprylylsilane, Dimethicone/PEG-10/15 Crosspolymer, Iodopropynyl Butylcarbamate, Ascorbyl Palmitate, Retinyl Palmitate
*Certified Organic
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
JACK BLACK INK BOOST TATTOO CARE
octinoxate, octisalate, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66738-381 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 3 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 8 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ETHYLHEXYL ISONONANOATE (UNII: I6KB4GE3K4) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) ASCORBYL PALMITATE (UNII: QN83US2B0N) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) LEONTOPODIUM NIVALE SUBSP. ALPINUM FLOWERING TOP (UNII: QQC1AK06RK) PEUCEDANUM OSTRUTHIUM LEAF (UNII: 86P27YRR6Y) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) BUDDLEJA DAVIDII LEAF (UNII: X380815D32) DIMETHICONE/PEG-10/15 CROSSPOLYMER (UNII: 21AS8B1BSS) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ARTEMISIA UMBELLIFORMIS FLOWER (UNII: 91OLL9AJ7D) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66738-381-40 44 mL in 1 KIT; Type 1: Convenience Kit of Co-Package 09/08/2020 06/01/2024 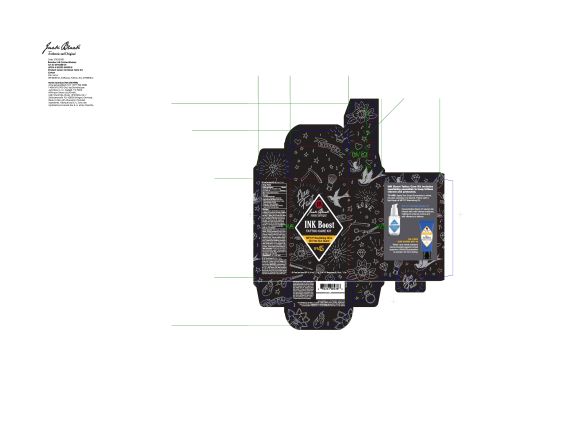
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/08/2020 06/01/2024 Labeler - Jack Black L.L.C (847024036) Establishment Name Address ID/FEI Business Operations Swiss American CDMO 080170933 manufacture(66738-381)

 Jack Black
Jack Black