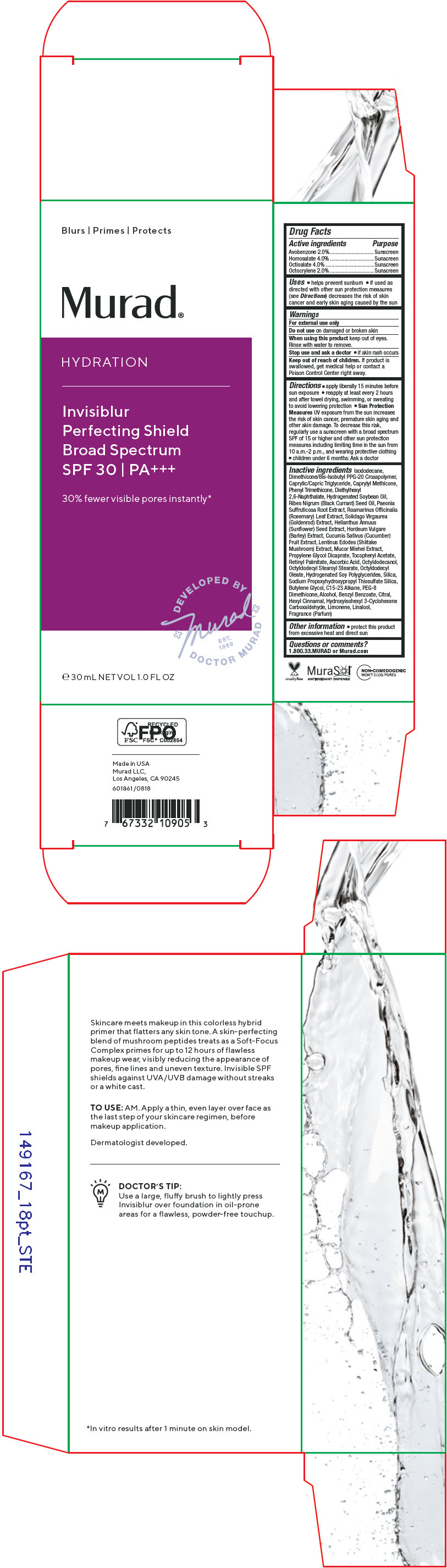
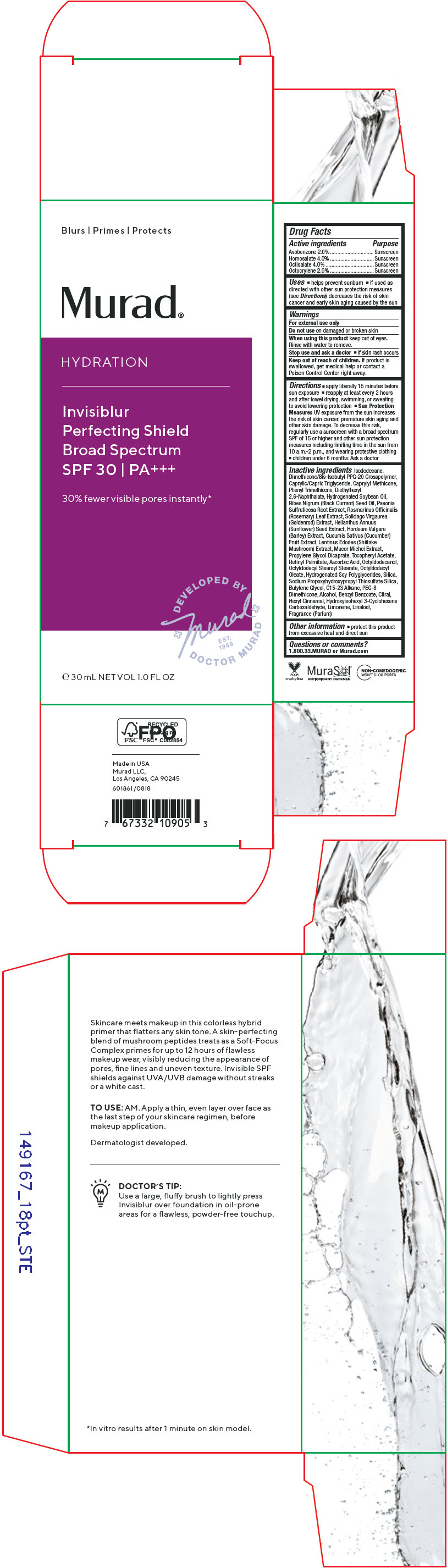
Label: HYDRATION INVISIBLUR PERFECTING SHIELD BROAD SPECTRUM SPF 30- avobenzone, homosalate, octisalate, octocrylene cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 70381-118-01, 70381-118-02 - Packager: Murad, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 9, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions) decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours and after towel drying, swimming, or sweating to avoid lowering protection
- Sun Protection Measures UV exposure from the sun increases the risk of skin cancer, premature skin aging and other skin damage. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including limiting time in the sun from 10 a.m.-2 p.m., and wearing protective clothing
- children under 6 months: Ask a doctor
-
Inactive ingredients
Isododecane, Dimethicone/Bis-Isobutyl PPG-20 Crosspolymer, Caprylic/Capric Triglyceride, Caprylyl Methicone, Phenyl Trimethicone, Diethylhexyl 2,6-Naphthalate, Hydrogenated Soybean Oil, Ribes Nigrum (Black Currant) Seed Oil, Paeonia Suffruticosa Root Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract, Solidago Virgaurea (Goldenrod) Extract, Helianthus Annuus (Sunflower) Seed Extract, Hordeum Vulgare (Barley) Extract, Cucumis Sativus (Cucumber) Fruit Extract, Lentinus Edodes (Shiitake Mushroom) Extract, Mucor Miehei Extract, Propylene Glycol Dicaprate, Tocopheryl Acetate, Retinyl Palmitate, Ascorbic Acid, Octyldodecanol, Octyldodecyl Stearoyl Stearate, Octyldodecyl Oleate, Hydrogenated Soy Polyglycerides, Silica, Sodium Propoxyhydroxypropyl Thiosulfate Silica, Butylene Glycol, C15-23 Alkane, PEG-8 Dimethicone, Alcohol, Benzyl Benzoate, Citral, Hexyl Cinnamal, Hydroxyisohexyl 3-Cyclohexene Carboxaldehyde, Limonene, Linalool, Fragrance (Parfum)
- Other information
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
HYDRATION INVISIBLUR PERFECTING SHIELD BROAD SPECTRUM SPF 30
avobenzone, homosalate, octisalate, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70381-118 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 4 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2 g in 100 mL Inactive Ingredients Ingredient Name Strength ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/BIS-ISOBUTYL PPG-20 CROSSPOLYMER (UNII: O4I3UFO6ZF) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) DIETHYLHEXYL 2,6-NAPHTHALATE (UNII: I0DQJ7YGXM) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) RIBES NIGRUM SEED OIL (UNII: GKE1188837) PAEONIA X SUFFRUTICOSA ROOT (UNII: 7M7E9A2C8J) ROSEMARY (UNII: IJ67X351P9) SUNFLOWER SEED (UNII: R9N3379M4Z) CUCUMBER (UNII: YY7C30VXJT) PROPYLENE GLYCOL DICAPRATE (UNII: U783H9JHWY) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ASCORBIC ACID (UNII: PQ6CK8PD0R) OCTYLDODECANOL (UNII: 461N1O614Y) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) OCTYLDODECYL OLEATE (UNII: MCA43PK7MH) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM PROPOXYHYDROXYPROPYL THIOSULFATE SILICA (UNII: 208G222332) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) C15-23 ALKANE (UNII: J3N6X3YK96) PEG-8 DIMETHICONE (UNII: GIA7T764OD) ALCOHOL (UNII: 3K9958V90M) BENZYL BENZOATE (UNII: N863NB338G) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) SOLIDAGO VIRGAUREA FLOWERING TOP (UNII: 5405K23S50) SHIITAKE MUSHROOM (UNII: 1A64QN2D2F) MUCOR PLUMBEUS (UNII: D7401PWY6E) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) HORDEUM VULGARE WHOLE (UNII: 8JBE478M5Q) Product Characteristics Color YELLOW (Clear to slightly yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70381-118-02 1 in 1 CARTON 08/30/2021 1 NDC:70381-118-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/30/2021 Labeler - Murad, LLC (781254792) Establishment Name Address ID/FEI Business Operations Cosway Company, Inc. 052400223 MANUFACTURE(70381-118)