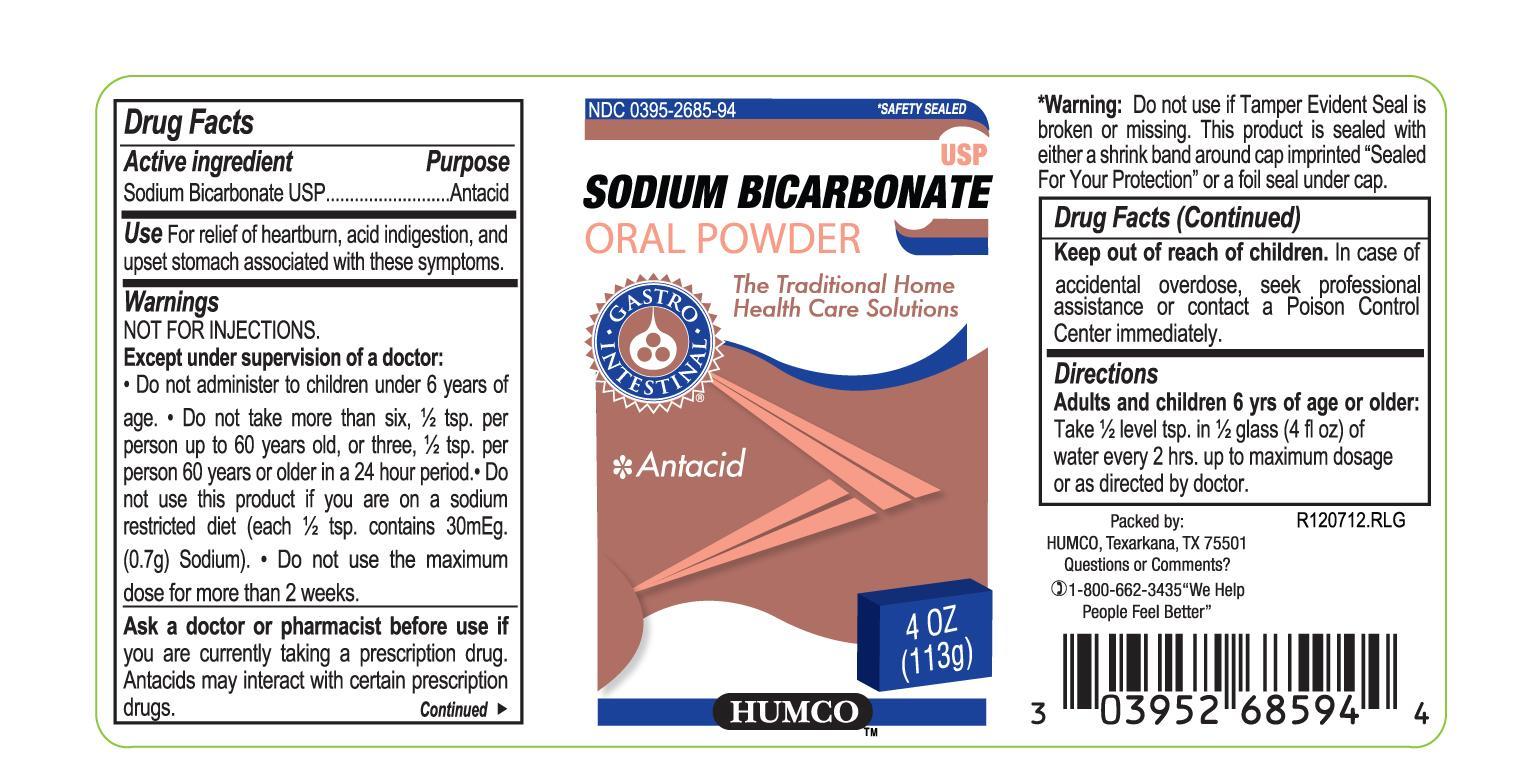
Label: HUMCO SODIUM BICARBONATE- sodium bicarbonate powder
- NDC Code(s): 0395-2685-01, 0395-2685-94
- Packager: Humco Holding Group, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Use
-
Warnings
NOT FOR INJECTIONS
Except under supervision of a doctor do not administer to children under 6 years of age.
Do not take more than six, 1/2 tsp. per person up to 60 years old, or three 1/2 tsp. per person 60 years or older in a 24 hour period.
Do not use this product if you are on a sodium restricted diet (each 1/2 tsp. contains 30 mEq (0.7 g) Sodium).
Do not use the maximum does more than 2 weeks.
- Ask a doctor or pharmacist before use if
- Keep out of reach of children.
- Directions
- Inactive ingredients
- Packae Principal Display Pannel
- Principal Display Pannel
- New Label
-
INGREDIENTS AND APPEARANCE
HUMCO SODIUM BICARBONATE
sodium bicarbonate powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0395-2685 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM BICARBONATE 1000 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0395-2685-94 113 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/14/2017 2 NDC:0395-2685-01 454 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/14/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 03/25/1998 Labeler - Humco Holding Group, Inc. (825672884) Registrant - Pharma Nobis, LLC (118564114) Establishment Name Address ID/FEI Business Operations Pharma Nobis, LLC 118564114 label(0395-2685) , manufacture(0395-2685) , pack(0395-2685) , analysis(0395-2685)