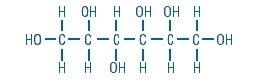Label: SORBITOL irrigant
- NDC Code(s): 0338-0295-47
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 31, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
3% Sorbitol Urologic Irrigating Solution is a sterile, nonpyrogenic, nonhemolytic, electrically nonconductive solution in a single dose UROMATIC Plastic Container for use as a urologic irrigating solution. Each liter contains 30 g Sorbitol in Water for Injection. pH 5.0 (4.5 to 6.5). Osmolarity 165 mOsmol/L (calc.). No antimicrobial agent has been added.
Sorbitol is a reduced form of dextrose and is designated chemically as D-Glucitol,
The UROMATIC plastic container is fabricated from a specially formulated polyvinyl chloride (PL 146 plastic). The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexyl phthalate (DEHP), up to 5 parts per million; however, the safety of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as by tissue culture toxicity studies.
-
CLINICAL PHARMACOLOGY
3% Sorbitol Urologic Irrigating Solution is useful as an irrigating fluid for the urinary bladder because the sorbitol solution is nonhemolytic, electrically nonconductive, and provides a high degree of visibility for urologic procedures requiring endoscopy. During transurethral surgical procedures, the solution acts as a lavage for removing blood and tissue fragments. It is also useful as an irrigating fluid to maintain the patency of an indwelling catheter in the immediate postoperative period. If absorbed either intravascularly or extravascularly during transurethral resections, the sorbitol will be either metabolized to carbon dioxide and water via the fructose pathway, or excreted by a normally functioning kidney.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Not for injection.
Not for use in patients with documented or suspected fructose intolerance.
Solutions for urologic irrigation must be used with caution in patients with severe cardiopulmonary or renal dysfunction.
Irrigating fluids used during transurethral prostatectomy have been demonstrated to enter the systemic circulation in relatively large volumes; thus sorbitol irrigating solution must be regarded as a systemic drug. Absorption of large amounts of fluids containing sorbitol may significantly alter cardiopulmonary and renal dynamics. Appropriate patient monitoring should be maintained due to the possibility of fluid overload. Should fluid overload occur, intensive fluid and electrolyte management is necessary. Monitoring of fluid and electrolyte levels beyond the acute phase may be considered due to the possibility of delayed fluid absorption. (See ADVERSE REACTIONS, Post-Marketing Experience)
Hyperglycemia from metabolism of absorbed sorbitol may occur in patients with diabetes mellitus.
The contents of an opened container should be used promptly to minimize the possibility of bacterial growth or pyrogen formation. Discard the unused portion of irrigating solution since no antimicrobial agent has been added.
-
PRECAUTIONS
The cardiovascular status, especially of the patient with cardiac disease, should be carefully observed before and during transurethral resection of the prostate when using 3% Sorbitol Urologic Irrigating Solution, because the quantity of fluid absorbed into the systemic circulation by opened prostatic veins may produce significant expansion of the intravascular fluid and lead to fulminating congestive heart failure.
Shift of sodium free intracellular fluid into the extracellular compartment, following systemic absorption may lower serum concentration and aggravate preexisting hyponatremia.
Excessive loss of water and electrolytes may lead to serious imbalances. With continuous administration of solution, loss of water may occur in excess of electrolytes, producing hypernatremia.
Sustained diuresis that results from transurethral irrigation with sorbitol irrigating solutions may obscure and intensify inadequate hydration or hypovolemia.
Pregnancy
Teratogenic Effects
Animal reproduction studies have not been conducted with 3% Sorbitol Urologic Irrigating Solution. It is not known whether 3% Sorbitol Urologic Irrigating Solution can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. 3% Sorbitol Urologic Irrigating Solution should be given to a pregnant woman only if clearly needed.
Geriatric Use
Clinical studies of Irrigation Solutions did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from other younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Do not administer unless the solution is clear and the seal is intact.
-
ADVERSE REACTIONS
Life threatening adverse reactions with intravenous sorbitol infusions have been reported in patients with fructose intolerance.
The literature reports occasional adverse reactions for intravenous sorbitol infusions. These include disturbances such as acidosis, electrolyte loss, marked diuresis, urinary retention, edema, dryness of mouth and thirst, and dehydration; cardiovascular/pulmonary disorders such as pulmonary congestion, hypotension, tachycardia, angina-like pains, and other general reactions such as blurred vision, convulsions, nausea, vomiting, diarrhea, rhinitis, chills, vertigo, and backache. Allergic reactions reported to occur from sorbitol include urticaria.
Should adverse reactions occur, discontinue the irrigant and reevaluate the clinical status of the patient.
-
DOSAGE AND ADMINISTRATION
The volume of solution needed will vary with the nature and duration of the urologic procedure.
If desired, warm in overwrap to near body temperature in a water bath or oven heated to not more than 45°C.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
-
HOW SUPPLIED
3% Sorbitol Urologic Irrigating Solution in UROMATIC plastic containers is available as follows:
2B7357
3000mL
NDC 0338-0295-47
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended the product be stored at room temperature (25°C); brief exposure up to 40°C does not adversely affect the product.
-
DIRECTIONS FOR USE
Tear overwrap down side at slit and remove solution container. Visually inspect the container. If the outlet port protector is damaged, detached, or not present, discard container as solution path sterility may be impaired. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. Check for minute leaks by squeezing bag firmly. If leaks are found, discard solution as sterility may be impaired.
Use Aseptic Technique.
- 1.
- Suspend container using hanger hole.
- 2.
- Remove protector from outlet port.
- 3.
- Attach irrigation set. Refer to complete directions accompanying set.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - PACKAGING LABELING
Container Label
LOT
EXP3000 mL
2B7357
NDC 0338-0295-47NOT FOR INJECTION
3%
Sorbitol Urologic
Irrigating SolutionEACH 100 mL CONTAINS 3 g SORBITOL
NO ANTIMICROBIAL AGENT HAS BEEN ADDED
pH 5.0 (4.5 TO 6.5) HYPOTONIC
OSMOLARITY 165 mOsmol/L (CALC)
STERILE NONPYROGENIC SINGLE DOSE
CONTAINERDO NOT USE UNLESS SOLUTION IS CLEAR
DISCARD UNUSED PORTION HYPOTONIC
SOLUTION FOR BLADDER IRRIGATION IN
CONNECTION WITH CYSTOSCOPIC EXAMINATION
AND UROLOGICAL SURGICAL PROCEDURES SEE
DIRECTION SHEETCAUTIONS SQUEEZE AND INSPECT INNER BAG
WHICH MAINTAINS PRODUCT STERILITY
DISCARD IF LEAKS ARE FOUND RX ONLY
STORE UNIT IN MOISTURE BARRIER OVERWRAP
AT ROOM TEMPERATURE (25°C) UNTIL READY TO
USE AVOID EXCESSIVE HEAT SEE INSERTUROMATIC CONTAINER
PL 146 PLASTIC
BAXTER UROMATIC AND PL 146 ARE
TRADEMARKS OF BAXTER INTERNATIONAL INCBaxter Logo
BAXTER HEALTHCARE CORPORATION
DEERFIELD IL 60015 USAMADE IN USA
FOR PRODUCT INFORMATION
1-800-933-0303 -
INGREDIENTS AND APPEARANCE
SORBITOL
sorbitol irrigantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-0295 Route of Administration IRRIGATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SORBITOL (UNII: 506T60A25R) (SORBITOL - UNII:506T60A25R) SORBITOL 30.00 g in 1000.00 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-0295-47 3000 mL in 1 BAG; Type 0: Not a Combination Product 05/30/1980 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017863 05/30/1980 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 059140764 MANUFACTURE(0338-0295) , ANALYSIS(0338-0295) , PACK(0338-0295) , LABEL(0338-0295) , STERILIZE(0338-0295) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 194684502 ANALYSIS(0338-0295)