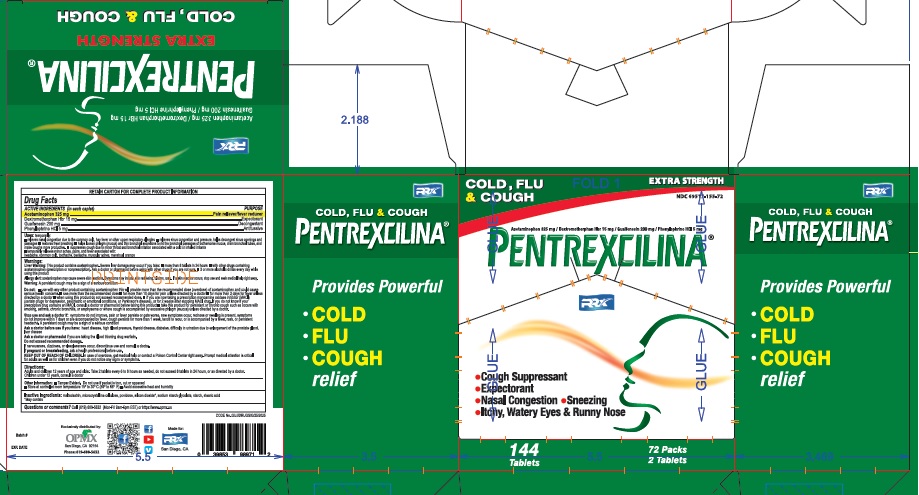
Label: PENTREXCILINA- acetaminophen, phenylephrine hydrochloride, dextromethorphan hydrobromide tablet
- NDC Code(s): 69517-155-06, 69517-155-72
- Packager: Healthlife of USA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts
Active Ingredients (in each tablet)
Acetaminophen USP 325mg.........................................................Pain reliever/fever reducer
Guaifenesin USP 200mg..............................................................Expectorant
Phenylephrine HCL USP 5mg........................................................Decongestant
Dextromethorphan HBr USP 15mg................................................Antitussive
- Purpose
-
Uses
Temporarily
- Relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- Relieves sinus congestion and pressure, helps decongest sinus openings and passages
- Restores freer breathing
- Helps loosen bothersome mucus, drain bronchial tubes, and make coughs more productive
- Suppresses cough due to minor throat and bronchial irritation associated with a cold or inhaled irritants
- Temporarily relieves minor aches, pains and fever associated with: headache, common cold, toothache, backache, muscular aches, menstrual cramps
-
Warnings: Liver Warning:
This product contains acetaminophen. Severe liver damage may occur if you take:
- More than 8 tablets in 24 hours
- With other drugs containing acetaminophen (prescription or nonprescription). Ask a doctor or pharmacist before using with other drugs if you are not sure
- 3 or more alcoholic drinks every day while using this product
- Allergy alert:
-
Do not
- use with any other product containing acetaminophen this will provide more than the recommended dose (overdose) of acetaminophen and cold cause serious health concerns.
- use more than the recommended dose
- for more than 10 days for pain unless directed by a doctor
- for more than 3 days for fever unless directed by a doctor
- when using this product do not exceed recommended dose
- if you are now taking a presription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping MAOI drug. If you do not know if your prescription drug contains an MAOI, consult a doctor or pharmacist before taking this product
- take this product for persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema or where cough is accompanied by excessive phlegm (mucus) unless directed by a doctor.
-
Stop use and ask a doctor if:
symptoms do not improve, pain or fever persists or gets worse, new symptoms occur, redness or swelling is present, symptoms do not improve within 7 days or are accompanied by fever, cough persists for more than 1 week, tends to recur, or is accompanied by a fever, rash, or persistent headache. A persistent cough may be a sign of a serious condition
- Ask a doctor before use if you have:
- Ask a doctor or pharmacist if you are
- Do not exceed recommended dosage
- If pregnant or breast-feeding,
- KEEP OUT OF REACH OF CHILDREN
- Directions:
- Other Inforamtion:
- Inactive Ingredients
- Questions or comments?
- Pentrexcilina 2 Tablets in a pouch
-
INGREDIENTS AND APPEARANCE
PENTREXCILINA
acetaminophen, phenylephrine hydrochloride, dextromethorphan hydrobromide tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69517-155 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 15 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) Product Characteristics Color white Score 2 pieces Shape CAPSULE Size 16mm Flavor Imprint Code A15 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69517-155-06 6 in 1 PACKAGE 01/05/2018 1 2 in 1 POUCH; Type 0: Not a Combination Product 2 NDC:69517-155-72 72 in 1 PACKAGE 01/05/2018 2 2 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/05/2018 Labeler - Healthlife of USA (079656178) Establishment Name Address ID/FEI Business Operations Vovantis Laboratories Private Limited 650502151 manufacture(69517-155)