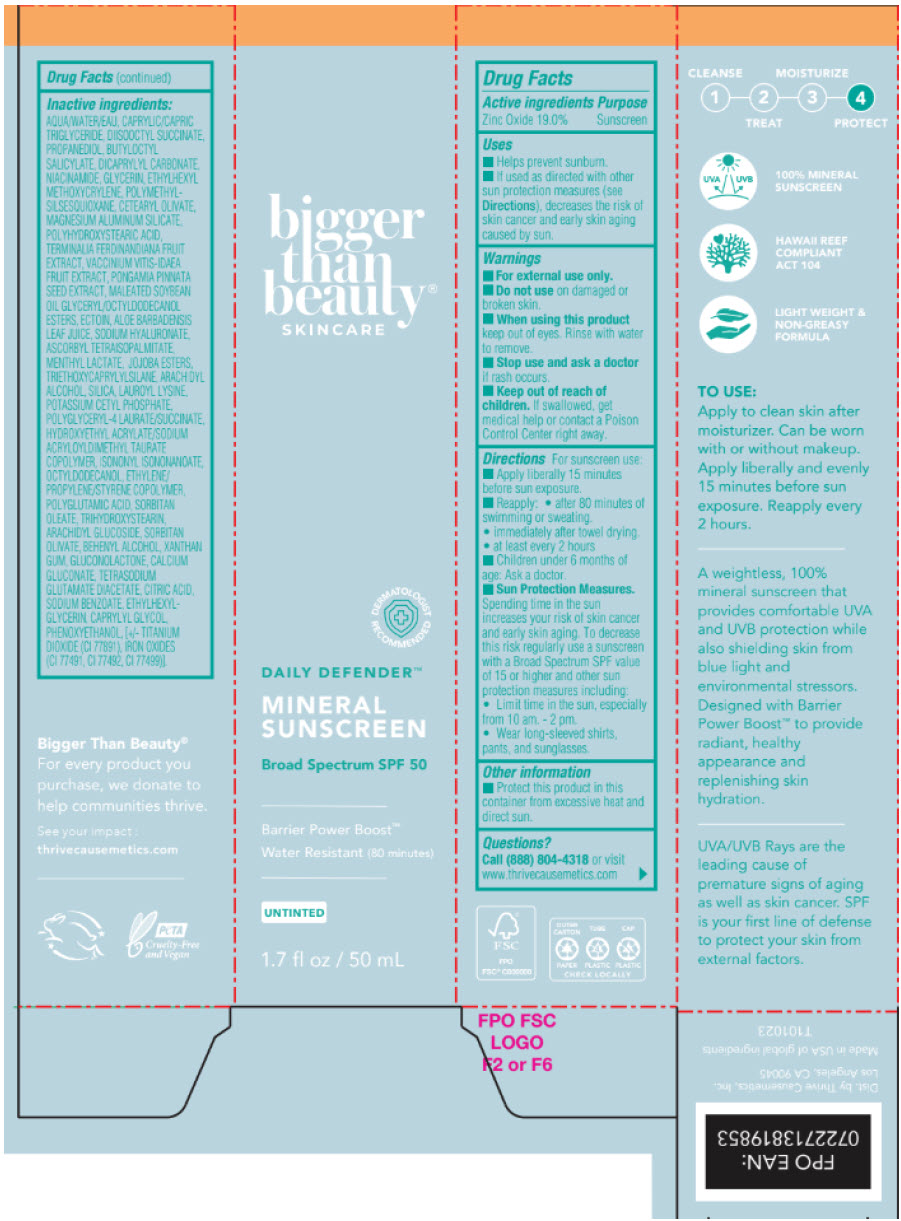
Label: DAILY DEFENDER MINERAL SUNSCREEN BROAD SPECTRUM SPF 50 WATER RESISTANT (80 MINUTES)- zinc oxide cream
- NDC Code(s): 77365-009-01
- Packager: Thrive Causemetics
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
-
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk Skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
For sunscreen use:
- Apply liberally 15 minutes before sun exposure.
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours.
- Children under 6 months of age: Ask a doctor.
-
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 am. - 2 pm.
- Wear long-sleeved shirts, pants, and sunglasses.
- Other information
- Questions?
-
Inactive ingredients
AQUA/WATER/EAU, CAPRYLIC/CAPRIC TRIGLYCERIDE, DIISOOCTYL SUCCINATE, PROPANEDIOL, BUTYLOCTYL SALICYLATE, DICAPRYLYL CARBONATE, NIACINAMIDE, GLYCERIN, ETHYLHEXYL METHOXYCRYLENE, POLYMETHYLSILSESQUIOXANE, CETEARYL OLIVATE, MAGNESIUM ALUMINUM SILICATE, POLYHYDROXYSTEARIC ACID, TERMINALIA FERDINANDIANA FRUIT EXTRACT, VACCINIUM VITIS-IDAEA FRUIT EXTRACT, PONGAMIA PINNATA SEED EXTRACT, MALEATED SOYBEAN OIL GLYCERYL/OCTYLDODECANOL ESTERS, ECTOIN, ALOE BARBADENSIS LEAF JUICE, SODIUM HYALURONATE, ASCORBYL TETRAISOPALMITATE, MENTHYL LACTATE, JOJOBA ESTERS, TRIETHOXYCAPRYLYLSILANE, ARACHIDYL ALCOHOL, SILICA, LAUROYL LYSINE, POTASSIUM CETYL PHOSPHATE, POLYGLYCERYL-4 LAURATE/SUCCINATE, HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER, ISONONYL ISONONANOATE, OCTYLDODECANOL, ETHYLENE/PROPYLENE/STYRENE COPOLYMER, POLYGLUTAMIC ACID, SORBITAN OLEATE, TRIHYDROXYSTEARIN, ARACHIDYL GLUCOSIDE, SORBITAN OLIVATE, BEHENYL ALCOHOL, XANTHAN GUM, GLUCONOLACTONE, CALCIUM GLUCONATE, TETRASODIUM GLUTAMATE DIACETATE, CITRIC ACID, SODIUM BENZOATE, ETHYLHEXYLGLYCERIN, CAPRYLYL GLYCOL, PHENOXYETHANOL, [+/- TITANIUM DIOXIDE (CI 77891), IRON OXIDES (CI 77491, CI 77492, CI 77499)].
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Box
-
INGREDIENTS AND APPEARANCE
DAILY DEFENDER MINERAL SUNSCREEN BROAD SPECTRUM SPF 50 WATER RESISTANT (80 MINUTES)
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:77365-009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 190 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOBUTYL SUCCINATE (UNII: 1241X4J800) PROPANEDIOL (UNII: 5965N8W85T) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) NIACINAMIDE (UNII: 25X51I8RD4) GLYCERIN (UNII: PDC6A3C0OX) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) CETEARYL OLIVATE (UNII: 58B69Q84JO) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) KAKADU PLUM (UNII: 0ZQ1D2FDLI) LINGONBERRY (UNII: 0UNK9RZQ7X) PONGAMIA PINNATA SEED (UNII: C2BRV53B1V) SOYBEAN OIL GLYCERETH-8 ESTERS (UNII: 8Y338J838M) ECTOINE (UNII: 7GXZ3858RY) ALOE VERA LEAF (UNII: ZY81Z83H0X) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ASCORBYL TETRAISOPALMITATE (UNII: 47143LT58A) MENTHYL LACTATE, (-)- (UNII: 2BF9E65L7I) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ARACHIDYL ALCOHOL (UNII: 1QR1QRA9BU) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LAUROYL LYSINE (UNII: 113171Q70B) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYGLYCERYL-4 LAURATE/SUCCINATE (UNII: KAB6DS9SBS) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) OCTYLDODECANOL (UNII: 461N1O614Y) STYRENE/ISOPRENE COPOLYMER (28:72; 210000 MW) (UNII: H58HX2GWJ5) POLYGLUTAMIC ACID (8000 MW) (UNII: D4JEG46UXV) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) ARACHIDYL GLUCOSIDE (UNII: 6JVW35JOOJ) SORBITAN OLIVATE (UNII: MDL271E3GR) DOCOSANOL (UNII: 9G1OE216XY) XANTHAN GUM (UNII: TTV12P4NEE) GLUCONOLACTONE (UNII: WQ29KQ9POT) CALCIUM GLUCONATE (UNII: SQE6VB453K) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM BENZOATE (UNII: OJ245FE5EU) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENOXYETHANOL (UNII: HIE492ZZ3T) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:77365-009-01 1 in 1 BOX 08/12/2024 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 08/12/2024 Labeler - Thrive Causemetics (039299574) Establishment Name Address ID/FEI Business Operations Cosmetic Solutions, LLC 807907928 MANUFACTURE(77365-009)