Label: PURPLEU BAOBAB REJUVENATING AMPOULE- niacinamide, adenosine liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 90069-205-01 - Packager: Purple U Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 2, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
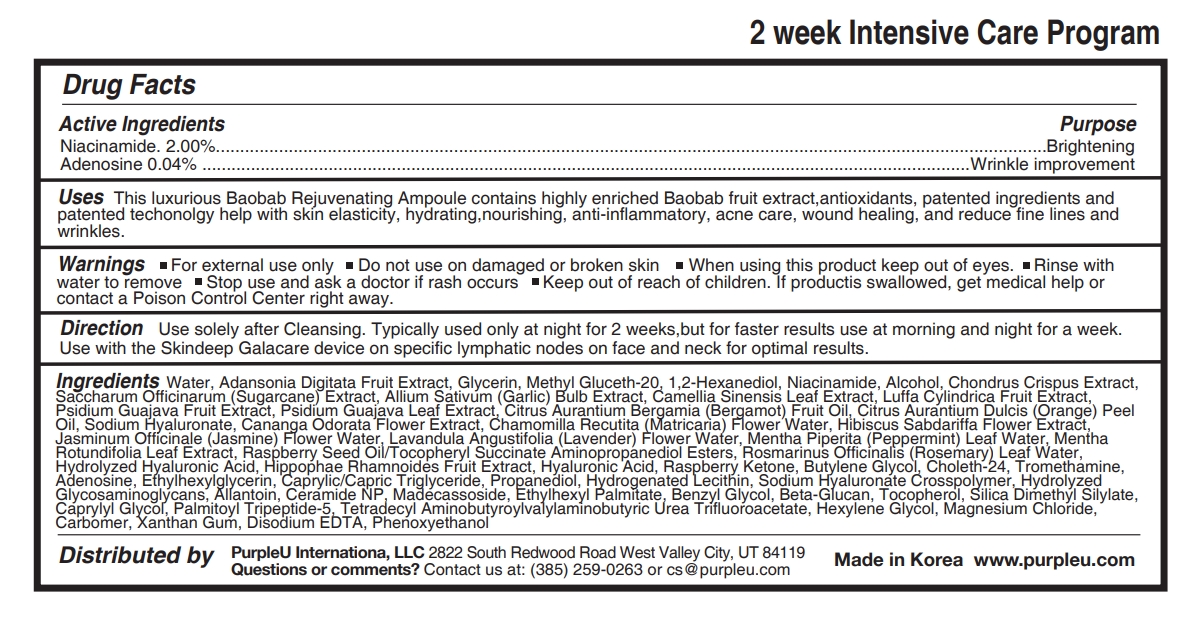
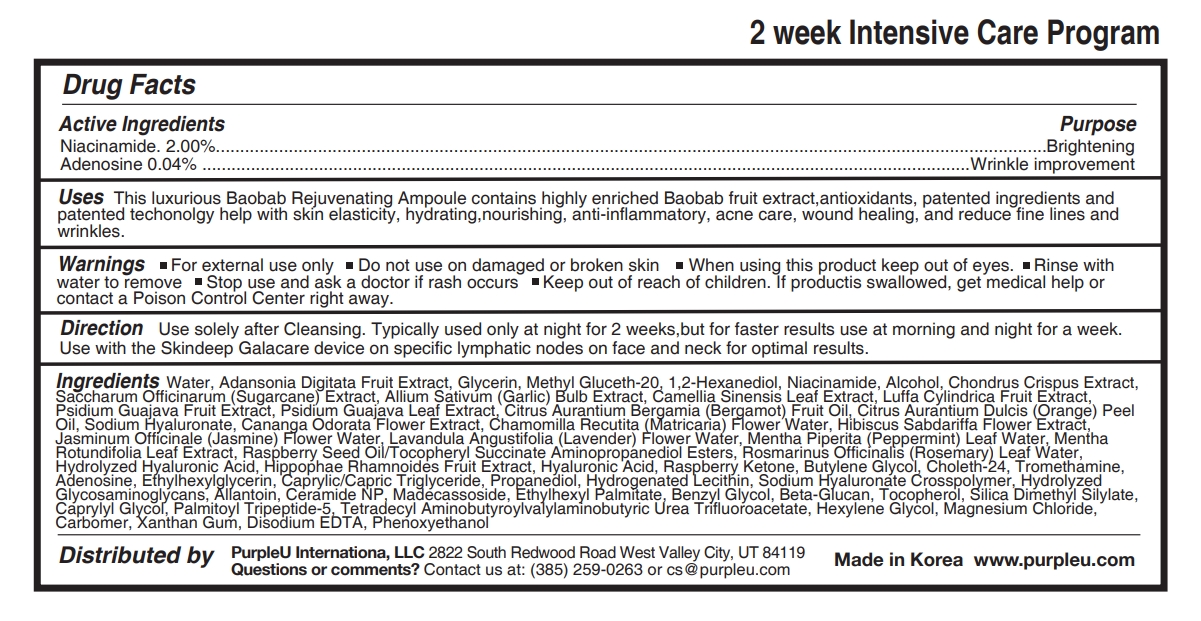
- Active Ingredients
- Purpose
- Uses
- Warnings
- Stop use and ask a doctor if
- Keep out of reach of children.
- Direction
-
Ingredients
Water, Adansonia Digitata Fruit Extract, Glycerin, Methyl Gluceth-20, 1,2-Hexanediol, Niacinamide, Alcohol, Chondrus Crispus Extract, Saccharum Officinarum (Sugarcane) Extract, Allium Sativum (Garlic) Bulb Extract, Camellia Sinensis Leaf Extract, Luffa Cylindrica Fruit Extract, Psidium Guajava Fruit Extract, Psidium Guajava Leaf Extract, Citrus Aurantium Bergamia (Bergamot) Fruit Oil, Citrus Aurantium Dulcis (Orange) Peel Oil, Sodium Hyaluronate, Cananga Odorata Flower Extract, Chamomilla Recutita (Matricaria) Flower Water, Hibiscus Sabdariffa Flower Extract, Jasminum Officinale (Jasmine) Flower Water, Lavandula Angustifolia (Lavender) Flower Water, Mentha Piperita (Peppermint) Leaf Water, Mentha Rotundifolia Leaf Extract, Raspberry Seed Oil/Tocopheryl Succinate Aminopropanediol Esters, Rosmarinus Officinalis (Rosemary) Leaf Water, Hydrolyzed Hyaluronic Acid, Hippophae Rhamnoides Fruit Extract, Hyaluronic Acid, Raspberry Ketone, Butylene Glycol, Choleth-24, Tromethamine, Adenosine, Ethylhexylglycerin, Caprylic/Capric Triglyceride, Propanediol, Hydrogenated Lecithin, Sodium Hyaluronate Crosspolymer, Hydrolyzed Glycosaminoglycans, Allantoin, Ceramide NP, Madecassoside, Ethylhexyl Palmitate, Benzyl Glycol, Beta-Glucan, Tocopherol, Silica Dimethyl Silylate, Caprylyl Glycol, Palmitoyl Tripeptide-5, Tetradecyl Aminobutyroylvalylaminobutyric Urea Trifluoroacetate, Hexylene Glycol, Magnesium Chloride, Carbomer, Xanthan Gum, Disodium EDTA, Phenoxyethanol
- Package Label
-
INGREDIENTS AND APPEARANCE
PURPLEU BAOBAB REJUVENATING AMPOULE
niacinamide, adenosine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:90069-205 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ADENOSINE (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) ADENOSINE 0.04 g in 100 mL NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 2 g in 100 mL Inactive Ingredients Ingredient Name Strength GARLIC (UNII: V1V998DC17) PSIDIUM GUAJAVA LEAF (UNII: PM0F263X0Y) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CANANGA ODORATA FLOWER (UNII: 76GTF6Z97M) CHAMOMILE FLOWER OIL (UNII: 60F80Z61A9) HIBISCUS SABDARIFFA FLOWER (UNII: 45TGG6IU6M) JASMINUM OFFICINALE FLOWER (UNII: 0Q8K841432) LAVENDER OIL (UNII: ZBP1YXW0H8) PEPPERMINT OIL (UNII: AV092KU4JH) MENTHA X ROTUNDIFOLIA LEAF (UNII: K59TXG2L3U) HIPPOPHAE RHAMNOIDES FRUIT (UNII: AVL0R9111T) 4-(P-HYDROXYPHENYL)-2-BUTANONE (UNII: 7QY1MH15BG) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CHOLETH-24 (UNII: 5UE7I54O43) TROMETHAMINE (UNII: 023C2WHX2V) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) TOCOPHEROL (UNII: R0ZB2556P8) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PALMITOYL TRIPEPTIDE-5 (UNII: 2A3916MQHO) HEXYLENE GLYCOL (UNII: KEH0A3F75J) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) XANTHAN GUM (UNII: TTV12P4NEE) PHENOXYETHANOL (UNII: HIE492ZZ3T) ROSEMARY OIL (UNII: 8LGU7VM393) HYALURONIC ACID (UNII: S270N0TRQY) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPANEDIOL (UNII: 5965N8W85T) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) HYDROLYZED GLYCOSAMINOGLYCANS (BOVINE; 50000 MW) (UNII: 997385V0VV) ALLANTOIN (UNII: 344S277G0Z) CERAMIDE 3 (UNII: 4370DF050B) MADECASSOSIDE (UNII: CQ2F5O6YIY) ETHYLHEXYL PALMITATE (UNII: 2865993309) ETHYLENE GLYCOL MONOBENZYL ETHER (UNII: 06S8147L47) TETRADECYL AMINOBUTYROYLVALYLAMINOBUTYRIC UREA TRIFLUOROACETATE (UNII: 0UBP26S1LG) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) GREEN TEA LEAF (UNII: W2ZU1RY8B0) LUFFA AEGYPTIACA FRUIT (UNII: QKL9NEO1F9) GUAVA (UNII: 74O70D6VG0) BERGAMOT OIL (UNII: 39W1PKE3JI) ORANGE OIL (UNII: AKN3KSD11B) WATER (UNII: 059QF0KO0R) ADANSONIA DIGITATA FRUIT (UNII: 51N9TR1W6P) GLYCERIN (UNII: PDC6A3C0OX) METHYL GLUCETH-20 (UNII: J3QD0LD11P) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALCOHOL (UNII: 3K9958V90M) CHONDRUS CRISPUS CARRAGEENAN (UNII: UE856F2T78) SUGARCANE (UNII: 81H2R5AOH3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:90069-205-01 28 mL in 1 AMPULE; Type 0: Not a Combination Product 09/03/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/03/2020 Labeler - Purple U Co., Ltd (694823532) Registrant - RECIPE CO., LTD (631158768) Establishment Name Address ID/FEI Business Operations RECIPE CO., LTD 631158768 manufacture(90069-205)