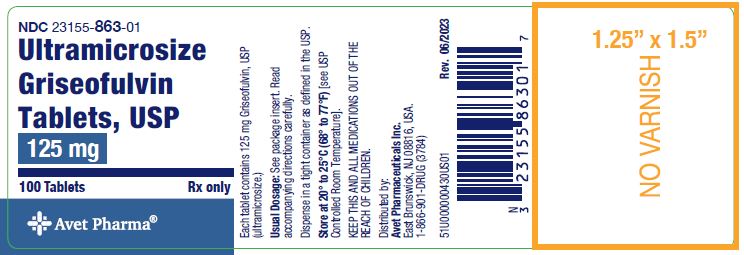
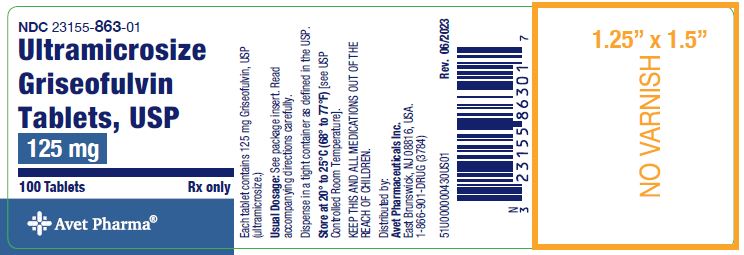
Label: ULTRAMICROSIZE GRISEOFULVIN- griseofulvin tablet
- NDC Code(s): 23155-863-01, 23155-864-01
- Packager: Heritage Pharmaceuticals Inc d/b/a Avet Pharmaceuticals Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Ultramicrosize griseofulvin tablets, USP contain ultramicrosize crystals of griseofulvin, an antibiotic derived from a species of Penicillium. Griseofulvin crystals are partly dissolved in polyethylene glycol 8000 and partly dispersed throughout the tablet matrix.
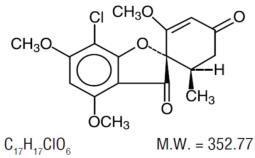
The chemical name of griseofulvin, USP is 7-Chloro-2’,4,6-trimethoxy-6’β-methylspiro[benzofuran-2( 3H),1’-[2]cyclohexene]-3,4’-dione. Its structural formula is:
Griseofulvin, USP occurs as a white to creamy white, odorless powder which is very slightly soluble in water, soluble in acetone, dimethylformamide, and chloroform and sparingly soluble in alcohol.
Each ultramicrosize griseofulvin tablet contains 125 mg or 250 mg griseofulvin ultramicrosize.
The inactive ingredients for ultramicrosize griseofulvin tablets, 125 mg or 250 mg, include: corn starch, lactose anhydrous, magnesium stearate, polyethylene glycol 8000, and sodium lauryl sulfate.
-
CLINICAL PHARMACOLOGY
Microbiology
- Griseofulvin is fungistatic with in vitro activity against various species of Microsporum, Epidermophyton, and Trichophyton. It has no effect on bacteria or other genera of fungi.
Pharmacokinetics
– Following oral administration, griseofulvin is deposited in the keratin precursor cells and has a greater affinity for diseased tissue. The drug is tightly bound to the new keratin which becomes highly resistant to fungal invasions.
The efficiency of gastrointestinal absorption of ultramicrocrystalline griseofulvin is approximately one and one-half times that of the conventional microsize griseofulvin. This factor permits the oral intake of two-thirds as much ultramicrocrystalline griseofulvin as the microsize form. However, there is currently no evidence that this lower dose confers any significant clinical differences with regard to safety and/or efficacy.
In a bioequivalence study conducted in healthy volunteers (N=24) in the fasted state, 250 mg ultramicrocrystalline griseofulvin tablets were compared with 250 mg ultramicrocrystalline griseofulvin tablets that were physically altered (crushed) and administered with applesauce. The 250 mg ultramicrocrystalline griseofulvin tablets were found to be bioequivalent to the physically altered (crushed) 250 mg ultramicro-crystalline griseofulvin tablets (See Table 1).
Table 1: Mean (±SD) of the Pharmacokinetic Parameters for Griseofulvin administered in applesauce as a Single-Dose of Ultramicrocrystalline Griseofulvin Tablets 250-mg Uncrushed and Crushed to fasted Healthy Volunteers (N=24) 250 mg
Ultramicrocrystalline
Griseofulvin
Tablets
Unaltered250 mg
Ultramicrocrystalline
Griseofulvin
Tablets Physically
Altered
(Crushed and
in Applesauce)Cmax
(ng/mL)600.61 (± 167.6) 672.61 (± 146.2) Tmax (hr) 4.04 (± 2.2) 3.08 (± 1.02) AUC
(ng.hr/mL)8,618.89 (± 1,907.2) 9,023.71 (± 1,911.5) -
INDICATIONS AND USAGE
Ultramicrosize griseofulvin tablets are indicated for the treatment of the following ringworm infections; tinea corporis (ringworm of the body), tinea pedis (athlete’s foot), tinea cruris (ringworm of the groin and thigh), tinea barbae (barber’s itch), tinea capitis (ringworm of the scalp), and tinea unguium (onychomycosis, ringworm of the nails), when caused by one or more of the following genera of fungi: Trichophyton rubrum, Trichophyton tonsurans, Trichophyton mentagrophytes, Trichophyton interdigitalis,Trichophyton verrucosum, Trichophyton megnini, Trichophyton gallinae, Trichophyton crateriform, Trichophyton sulphureum, Trichophyton schoenleini, Microsporum audouini, Microsporum canis, Microsporum gypseum and Epidermophyton floccosum. NOTE: Prior to therapy, the type of fungi responsible for the infection should be identified. The use of the drug is not justified in minor or trivial infections which will respond to topical agents alone. Griseofulvin is not effective in the following: bacterial infections, candidiasis (moniliasis), histoplasmosis, actinomycosis, sporotrichosis, chromoblastomycosis, coccidioidomycosis, North American blastomycosis, cryptococcosis (torulosis), tinea versicolor and nocardiosis.
-
CONTRAINDICATIONS
Two cases of conjoined twins have been reported since 1977 in patients taking griseofulvin during the first trimester of pregnancy. Griseofulvin should not be prescribed to pregnant patients. If the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
This drug is contraindicated in patients with porphyria or hepatocellular failure and in individuals with a history of hypersensitivity to griseofulvin.
- WARNINGS
-
Serious Skin Reactions
Severe skin reactions (e.g. Stevens-Johnson syndrome, toxic epidermal necrolysis) and erythema multiforme have been reported with griseofulvin use. These reactions may be serious and may result in hospitalization or death. If severe skin reactions occur, griseofulvin should be discontinued (see ADVERSE REACTIONSsection).
-
Hepatotoxicity
Elevations in AST, ALT, bilirubin, and jaundice have been reported with griseofulvin use. These reactions may be serious and may result in hospitalization or death. Patients should be monitored for hepatic adverse events and discontinuation of griseofulvin considered if warranted (see ADVERSE REACTIONSsection).
Animal Toxicology
– Chronic feeding of griseofulvin, at levels ranging from 0.5% to 2.5% of the diet resulted in the development of liver tumors in several strains of mice, particularly in males. Smaller particle sizes result in an enhanced effect. Lower oral dosage levels have not been tested. Subcutaneous administration of relatively small doses of griseofulvin once a week during the first three weeks of life has also been reported to induce hepatomata in mice. Thyroid tumors, mostly adenomas but some carcinomas, have been reported in male rats receiving griseofulvin at levels of 2.0%, 1.0% and 0.2% of the diet, and in female rats receiving the two higher dose levels. Although studies in other animal species have not yielded evidence of tumorigenicity, these studies were not of adequate design to form a basis for conclusion in this regard. In subacute toxicity studies, orally administered griseofulvin produced hepatocellular necrosis in mice, but this has not been seen in other species. Disturbances in porphyrin metabolism have been reported in griseofulvin-treated laboratory animals. Griseofulvin has been reported to have a colchicine-like effect on mitosis and cocarcinogenicity with methylcholanthrene in cutaneous tumor induction in laboratory animals.
Usage in Pregnancy: see CONTRAINDICATIONSsection.
Animal Reproduction Studies
– It has been reported in the literature that griseofulvin was found to be embryotoxic and teratogenic on oral administration to pregnant rats. Pups with abnormalities have been reported in the litters of a few bitches treated with griseofulvin. Suppression of spermatogenesis has been reported to occur in rats, but investigation in man failed to confirm this.
-
PRECAUTIONS
Patients on prolonged therapy with any potent medication should be under close observation. Periodic monitoring of organ system function, including renal, hepatic, and hematopoietic, should be done. Since griseofulvin is derived from species of Penicillium, the possibility of cross sensitivity with penicillin exists; however, known penicillin-sensitive patients have been treated without difficulty. Since a photosensitivity reaction is occasionally associated with griseofulvin therapy, patients should be warned to avoid exposure to intense natural or artificial sunlight. Lupus erythematosus or lupus-like syndromes, have been reported in patients receiving griseofulvin. Griseofulvin decreases the activity of warfarin-type anticoagulants so that patients receiving these drugs concomitantly may require dosage adjustment of the anticoagulant during and after griseofulvin therapy. Barbiturates usually depress griseofulvin activity, and concomitant administration may require a dosage adjustment of the antifungal agent. There have been reports in the literature of possible interactions between griseofulvin and oral contraceptives. The effect of alcohol may be potentiated by griseofulvin, producing such effects as tachycardia and flush.
-
ADVERSE REACTIONS
There have been post-marketing reports of severe skin and hepatic adverse events associated with griseofulvin use (see WARNINGSsection).
When adverse reactions occur, they are most commonly of the hypersensitivity type such as skin rashes, urticaria, erythema multiforme-like drug reactions, and rarely, angioneurotic edema, and may necessitate withdrawal of therapy and appropriate countermeasures. Paresthesia of the hands and feet have been reported after extended therapy. Other side effects reported occasionally are oral thrush, nausea, vomiting, epigastric distress, diarrhea, headache, fatigue, dizziness, insomnia, mental confusion, and impairment of performance of routine activities. Proteinuria and leukopenia have been reported rarely. Administration of the drug should be discontinued if granulocytopenia occurs. When rare, serious reactions occur with griseofulvin, they are usually associated with high dosages, long periods of therapy, or both.
To report SUSPECTED ADVERSE REACTIONS, contact Avet Pharmaceuticals Inc., at 1-866-901-DRUG (3784) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
DOSAGE AND ADMINISTRATION
Accurate diagnosis of infecting organism is essential. Identification should be made either by direct microscopic examination of a mounting of infected tissue in a solution of potassium hydroxide or by culture on an appropriate medium. Medication must be continued until the infecting organism is completely eradicated as indicated by appropriate clinical or laboratory examination. Representative treatment periods are tinea capitis, 4 to 6 weeks; tinea corporis, 2 to 4 weeks; tinea pedis, 4 to 8 weeks; tinea unguium–depending on rate of growth–fingernails, at least 4 months; toenails, at least 6 months.
General measures in regard to hygiene should be observed to control sources of infection or reinfection. Concomitant use of appropriate topical agents is usually required, particularly in treatment of tinea pedis. In some forms of athlete’s foot, yeasts and bacteria may be involved as well as fungi. Griseofulvin will not eradicate the bacterial or monilial infection. Ultramicrosize griseofulvin tablets may be swallowed whole or crushed and sprinkled onto 1 tablespoonful of applesauce and swallowed immediately without chewing.
Adults: Daily administration of 375 mg (as a single dose or in divided doses) will give a satisfactory response in most patients with tinea corporis, tinea cruris, and tinea capitis. For those fungus infections more difficult to eradicate, such as tinea pedis and tinea unguium, a divided dose of 750 mg is recommended.
Pediatric Use: Approximately 7.3 mg per kg of body weight per day of ultramicrosize griseofulvin is an effective dose for most pediatric patients. On this basis, the following dosage schedule is suggested:
16 to 27 kg: 125 mg to 187.5 mg daily.
Over 27 kg: 187.5 mg to 375 mg daily
Children and infants 2 years of age and younger–dosage has not been established. Clinical experience with griseofulvin in children with tinea capitis indicates that a single daily dose is effective. Clinical relapse will occur if the medication is not continued until the infecting organism is eradicated.
-
HOW SUPPLIED
Ultramicrosize Griseofulvin Tablets, USP 125 mg, are supplied as off white, round tablets debossed with ‘CE’ over ‘3’ on one side and a functional score on the other side. They are available as follows:
Bottles of 100 NDC 23155-863-01.
Ultramicrosize Griseofulvin Tablets, USP 250 mg, are supplied as off white, round tablets debossed with ‘CE’ over ‘4’ on one side and a functional score on the other side. They are available as follows:
Bottles of 100 NDC 23155-864-01.
-
STORAGE
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP.
Manufactured by: Chartwell Pharmaceuticals, LLC.
Congers, NY 10920

Distributed by: Avet Pharmaceuticals Inc.
East Brunswick, NJ 08816
1.866.901.DRUG (3784)
Made in USA
51U000000433US02
Revised: 07/2023
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ULTRAMICROSIZE GRISEOFULVIN
griseofulvin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:23155-863 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GRISEOFULVIN (UNII: 32HRV3E3D5) (GRISEOFULVIN - UNII:32HRV3E3D5) GRISEOFULVIN 125 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color white Score 2 pieces Shape ROUND Size 10mm Flavor Imprint Code CE;3 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23155-863-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/30/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA061996 06/30/2023 ULTRAMICROSIZE GRISEOFULVIN
griseofulvin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:23155-864 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GRISEOFULVIN (UNII: 32HRV3E3D5) (GRISEOFULVIN - UNII:32HRV3E3D5) GRISEOFULVIN 250 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color white Score 2 pieces Shape ROUND Size 13mm Flavor Imprint Code CE;4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23155-864-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/30/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA061996 06/30/2023 Labeler - Heritage Pharmaceuticals Inc d/b/a Avet Pharmaceuticals Inc (780779901) Registrant - Chartwell Pharmaceuticals Congers, LLC. (118673447) Establishment Name Address ID/FEI Business Operations Chartwell Pharmaceuticals Congers, LLC. 118673447 analysis(23155-863, 23155-864) , manufacture(23155-863, 23155-864) , pack(23155-863, 23155-864) , label(23155-863, 23155-864)