Label: ZENRELIA- ilunocitinib tablet
-
NDC Code(s):
58198-5536-1,
58198-5536-2,
58198-5536-3,
58198-5537-1, view more58198-5537-2, 58198-5537-3, 58198-5538-1, 58198-5538-2, 58198-5538-3, 58198-5539-1, 58198-5539-2, 58198-5539-3
- Packager: Elanco US Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated September 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Zenrelia™
-
BOXED WARNING
(What is this?)
WARNING: VACCINE-INDUCED DISEASE AND INADEQUATE IMMUNE RESPONSE TO VACCINES
Based on results of the vaccine response study, dogs receiving Zenrelia are at risk of fatal vaccine-induced disease from modified live virus vaccines and inadequate immune response to any vaccine. Discontinue Zenrelia for at least 28 days to 3 months prior to vaccination and withhold Zenrelia for at least 28 days after vaccination (see Warnings and Target Animal Safety).
- DESCRIPTION
- INDICATIONS
-
DOSAGE AND ADMINISTRATION
The dose of Zenrelia (ilunocitinib tablets) is 0.27 to 0.36 mg ilunocitinib/lb (0.6 to 0.8 mg ilunocitinib/kg) body weight, administered orally, once daily, with or without food.
Dosing Chart
Weight Range
(in lb)Weight Range
(in kg)Number of Tablets to be Administered
4.8 mg tablets
6.4 mg tablets
8.5 mg tablets
15 mg tablets
6.6 – 8.8
3.0 - 4.0
0.5
8.9 – 11.8
4.1 – 5.3
0.5
11.9 – 14.3
5.4 – 6.5
0.5
14.4 – 17.7
6.6 – 8.0
1
17.8 – 23.6
8.1 – 10.6
1
23.7 – 31.1
10.7 – 14.1
1
31.2 – 35.4
14.2 – 16.0
1.5
35.5 – 43.1
16.1 – 19.5
1.5
43.2 – 55.0
19.6 – 24.9
1
55.1 – 62.5
25.0 – 28.3
2
62.6 – 83.3
28.4 – 37.4
1.5
83.4 – 110.0
37.5 – 49.9
2
110.1 – 137.5
50.0 – 62.4
2.5
137.6 – 166.0
62.5 – 74.9
3
≥ 166.1
≥ 75
Administer the appropriate combination
of tablet strengths -
WARNINGS
User Safety Warnings
Not for use in humans. Keep this drug out of the reach of children. Wash hands immediately after handling tablets. In case of accidental ingestion, seek medical attention immediately.
Animal Safety Warnings
Due to the risk of fatal vaccine-induced disease from modified live virus vaccines and inadequate immune response to any vaccine, including rabies vaccines, do not administer vaccines to a dog receiving Zenrelia. Discontinue Zenrelia for at least 28 days to 3 months prior to vaccination and withhold Zenrelia for at least 28 days after vaccination (see Target Animal Safety).
Dogs should be monitored for the development of infections because Zenrelia may increase susceptibility to opportunistic infections, including demodicosis, interdigital furunculosis, coccidiosis, and pneumonia, and exacerbation of subclinical or uncomplicated infections (see Target Animal Safety and Adverse Reactions).
Zenrelia is not for use in dogs with serious infections.
Zenrelia may cause a progressive or persistently decreased hematocrit, hemoglobin, and/or red blood cell count without a corresponding increase in absolute reticulocyte count (see Target Animal Safety).
New neoplastic conditions (benign and malignant) were observed in dogs treated with Zenrelia during clinical studies (see Adverse Reactions).
Consider the risks and benefits of treatment prior to initiating Zenrelia in dogs with a history of recurrent serious infections or recurrent demodicosis or neoplasia (see Adverse Reactions and Target Animal Safety).
Zenrelia modulates the immune system.
Zenrelia is not for use in dogs less than 12 months of age (see Target Animal Safety).
Keep Zenrelia in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
-
PRECAUTIONS
Dogs should be up to date on vaccinations prior to starting Zenrelia (see Target Animal Safety).
The safe use of Zenrelia has not been evaluated in breeding, pregnant, or lactating dogs.
Decreased prostate gland weights in intact male dogs were observed in a laboratory safety study (see Target Animal Safety).
The safe use of Zenrelia has not been evaluated in combination with glucocorticoids, cyclosporine, or other systemic immunosuppressive agents.
-
ADVERSE REACTIONS
Control of Atopic Dermatitis
In a masked field study assessing effectiveness and safety of Zenrelia for the control of atopic dermatitis in dogs, 181 Zenrelia-treated dogs and 87 placebo-treated dogs diagnosed with atopic dermatitis were evaluated for safety up to 112 days. By Day 112, 66.7% of placebo-treated dogs and 22.1% of Zenrelia-treated dogs exited the study. Adverse reactions seen during the field study are summarized in Table 1 below.
Table 1. Adverse Reactions through Day 112
N = number of dogs Adverse Reaction
Zenrelia N = 181
Number of Dogs (%)Placebo N = 87
Number of Dogs (%)Vomiting or nausea
40 (22.1 %)
14 (16.1 %)
Diarrhea
36 (19.9 %)
9 (10.3 %)
Lethargy
22 (12.2 %)
9 (10.3 %)
Otitis externa
19 (10.5 %)
20 (23.0 %)
Anorexia
17 (9.4 %)
7 (8.0 %)
Dermal growth (e.g., cyst, papilloma)
16 (8.8 %)
4 (4.6 %)
Epiphora or ocular discharge
14 (7.7 %)
1 (1.1 %)
Coughing or wheezing,
including respiratory infections12 (6.6 %)
2 (2.3 %)
Bacterial skin infection
10 (5.5 %)
9 (10.3 %)
Elevated liver enzyme(s)
10 (5.5 %)
2 (2.3 %)
Urinary tract infection
10 (5.5 %)
2 (2.3 %)
Upset stomach,
including flatulence and abdominal pain10 (5.5 %)
0
Leukopenia
9 (4.9 %)
1 (1.1 %)
Sneezing
8 (4.4 %)
1 (1.1 %)
Lipoma
7 (3.9 %)
1 (1.1 %)
Weight gain
7 (3.9 %)
0
Increased water intake
4 (2.2 %)
2 (2.3 %)
Gingivitis (occurrence or worsening)
4 (2.2 %)
0
Blood in stool
4 (2.2 %)
0
Elevated total bilirubin
4 (2.2 %)
0
Elevated triglyceride
4 (2.2 %)
0
Histiocytoma
3 (1.7 %)
0
Increased appetite
3 (1.7 %)
0
Fungal skin infection
3 (1.7 %)
2 (2.3 %)
Weight loss
2 (1.1 %)
1 (1.1 %)
Metastatic neoplasia (i.e., hemangiosarcoma)
1 (0.6 %)
0
Systemic fungal infection
1 (0.6 %)
0
Mast cell tumor
1 (0.6 %)
0
Abnormal hematology results likely related to Zenrelia treatment included thrombocytopenia, leukopenia, neutropenia, lymphopenia, eosinopenia, monocytopenia, and decreased red blood cell count.
Abnormal serum chemistry results likely related to Zenrelia treatment included increased hepatobiliary parameters (alanine transaminase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transferase, and total bilirubin), increased blood urea nitrogen (concurrently with an increase in creatinine for one dog), hypertriglyceridemia, hypercholesterolemia, hypoalbuminemia (without a concurrent hyperglobulinemia), and hypoglobulinemia (with or without a decrease in total protein).
Twelve Zenrelia-treated dogs withdrew from the study early due to an adverse reaction, nine of which were considered likely related to Zenrelia treatment. These reactions included repeated episodes of vomiting, leukopenia, neutropenia, worsening of pre-existing lymphocytosis, enlargement of a non-resolving histiocytoma, eyelid mass with bacterial blepharitis, otitis interna with vestibular disease, urinary tract infection, and upper respiratory infection. Five placebo dogs withdrew from the study early due to an adverse reaction (i.e., lethargy, worsening of pre-existing lymphocytosis, occurrence of nystagmus, skin infection, and teat infection).
One Zenrelia-treated dog was diagnosed with splenic and liver masses on Day 112. Histopathologic diagnosis after euthanasia one month later confirmed metastatic splenic and hepatic hemangiosarcoma. Another Zenrelia-treated dog experienced traumatic tendonitis and a puncture wound four days prior to study completion, which progressed to a serious infection. The owner elected amputation after study completion. A third Zenrelia-treated dog experienced a moderate neutropenia on Day 28 associated with a pre-existing subclinical urinary tract infection (UTI) that had progressed into a clinical UTI. The neutrophil count normalized seven days later while still receiving Zenrelia, prior to exiting the study to receive antibiotics.
Control of Pruritus Associated with Allergic Dermatitis
In a masked field study assessing effectiveness and safety of Zenrelia for the control of pruritus associated with allergic dermatitis in dogs, 206 Zenrelia-treated dogs and 100 placebo-treated dogs diagnosed with allergic dermatitis were evaluated for safety up to 112 days. By Day 112, 84% of placebo-treated dogs and 49.5% of Zenrelia-treated dogs exited the study. Adverse reactions seen during the field study are summarized in Table 2 below.
Table 2. Adverse Reactions through Day 112
N = number of dogs Adverse Reaction
Zenrelia N = 206
Number of Dogs (%)Placebo N = 100
Number of Dogs (%)Vomiting or nausea
32 (15.5%)
11 (11.0 %)
Diarrhea
26 (12.2 %)
5 (5.0 %)
Lethargy
25 (12.1 %)
7 (7.0 %)
Urinary tract infection
13 (6.3 %)
2 (2.0 %)
Anorexia
10 (4.9 %)
3 (3.0 %)
Coughing, wheezing, or difficulty breathing
9 (4.4 %)
0
Elevated liver enzyme(s)
8 (3.9 %)
0
Otitis externa
8 (3.9 %)
5 (5.0 %)
Increased water intake
7 (3.4 %)
2 (2.0 %)
Upset stomach, including flatulence,
retching, and abdominal pain5 (2.4 %)
4 (4.0 %)
Ocular discharge
5 (2.4 %)
1 (1.0 %)
Elevated triglyceride
5 (2.4 %)
0
Dermal or subcutaneous growth
(e.g., cyst, nodule)3 (1.5 %)
2 (2.0 %)
Sneezing
3 (1.5 %)
0
Blood in stool
3 (1.5 %)
0
Increased urination
3 (1.5 %)
0
Bacterial skin infection
2 (1.0 %)
4 (4.0 %)
Weight gain
2 (1.0 %)
0
Neurological disorder (e.g., tremors, ataxia)
2 (1.0 %)
0
Increased appetite
1 (0.5 %)
0
Fungal skin infection
1 (0.5 %)
0
Fever
1 (0.5 %)
0
Hematuria (without urinary tract infection)
1 (0.5 %)
0
Abnormal hematology results likely related to Zenrelia treatment included thrombocytosis, leukopenia, neutropenia, eosinopenia, and monocytopenia.
Abnormal serum chemistry results likely related to Zenrelia treatment included increased hepatobiliary parameters (alanine transaminase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transferase, and total bilirubin), increased blood urea nitrogen, increased creatinine, hypertriglyceridemia, hypercholesterolemia, hypoproteinemia, and hypoglobulinemia (with or without a decrease in total protein).
Seven Zenrelia-treated dogs withdrew from the study early due to an adverse reaction, four of which were considered likely related to Zenrelia treatment. These reactions included vomiting, lethargy, soft stool, neutropenia, increased liver enzymes, fever, abdominal discomfort, coughing, and wheezing. Four placebo treated dogs also withdrew from the study early due to an adverse reaction (i.e., splenic hemangiosarcoma, restlessness, abdominal pain, lethargy, and vomiting).
-
CONTACT INFORMATION
To report suspected adverse events, for technical assistance, or to obtain a copy of the Safety Data Sheet (SDS), contact Elanco US Inc at 1-888-545-5973.
For additional information about reporting adverse drug experiences for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Ilunocitinib is a non-selective JAK inhibitor which inhibits the function of a variety of pruritogenic, pro-inflammatory and allergy related cytokines that are dependent upon JAK enzymes. Ilunocitinib has a high potency for JAK1, JAK2, and tyrosine kinase 2 (TYK2) inhibition. Ilunocitinib is not a corticosteroid or an antihistamine.
Pharmacokinetics
Ilunocitinib is rapidly and well absorbed and excreted via the biliary/fecal route after oral administration in dogs. Following a single oral or intravenous administration of ilunocitinib at 0.8 mg/kg, the oral bioavailability based on area under the curve from the time of dosing to the last quantifiable plasma concentration (AUClast) was 80% in the fed state and 60% in the fasted state. The systemic clearance following intravenous administration was 399 mL/hour(h)/kg with a terminal half-life of 3.6 h. The volume of distribution was 1390 mL/kg (n=8). The maximum plasma concentration (Cmax) and AUClast were 120% and 45% higher, respectively, in the fed state as compared to the fasted state (n=16).
In a laboratory margin of safety study, healthy adult dogs (see Target Animal Safety) received daily oral administration of Zenrelia at 0.8 mg/kg, 1.6 mg/kg, 2.4 mg/kg, or 4.0 mg/kg for 182 consecutive days. Dogs were dosed in the fed state, the prandial state of maximum bioavailability. At 0.8 mg/kg, the ilunocitinib mean (coefficient of variation %) Cmax was 310 ng/mL (20.6%) with a median time to Cmax of 2 h (range 1 – 2 h), and the AUClast and half-life were 1360 h*ng/mL (25.1%) and 3.3 h (11.9%), respectively. Minimal accumulation was observed between Days 1 and 182 with geometric mean accumulation ratios for Cmax and AUClast between 1.1 and 1.6. After the first dose, Cmax increased in a linear but less than proportional manner where a 5-fold increase in dose resulted in a 3.4-fold (90% confidence limit: 2.9 – 4.0) increase in Cmax. There was a non-linear relationship between dose and AUClast where a 5-fold increase in dose resulted in a 4.2-fold (90% confidence limit: 3.4 – 5.1) increase in AUClast. Pharmacokinetic parameters are presented as geometric means.
-
EFFECTIVENESS
Control of Atopic Dermatitis:
A masked, placebo-controlled field study was conducted at 25 veterinary clinics in the US and Canada, enrolling 268 client-owned dogs diagnosed with atopic dermatitis and having at least moderate pruritus and mild skin lesions. Dogs were randomized to once daily treatment with Zenrelia at 0.6 – 0.8 mg/kg or placebo, at a ratio of 2:1 respectively. Other medications that could affect the evaluation of effectiveness were not allowed during the study, such as corticosteroids, antihistamines, and cyclosporine. Treatment success for each dog was defined as a ≥ 50% reduction from baseline in owner-assessed pruritus scores on the Pruritus Visual Analog Scale (PVAS) or a ≥ 50% reduction from baseline in veterinarian-assessed skin lesion scores on the Canine Atopic Dermatitis Extent and Severity Index version 4 (CADESI-4) on Day 28. The proportion of dogs in the Zenrelia group that were treatment successes was greater than and significantly different from the placebo group on Day 28 (Table 3, below).
Table 3. Estimated Proportion of Dogs Achieving Treatment Success
* Based on back-transformed least squares means. † Placebo vs. Zenrelia p < 0.001 N = Number of dogs Treatment Group
Estimated Proportion of Success*
Zenrelia (N = 172)
0.83
Placebo (N = 77)
0.31†
The Zenrelia group had a higher proportion of dogs with a ≥ 50% reduction from baseline in both owner-assessed PVAS and veterinarian-assessed CADESI-4 scores, compared to placebo, at all time points. The mean owner-assessed PVAS and veterinarian-assessed CADESI-4 scores were also lower for the Zenrelia group compared to the placebo group at all time points.
Control of Pruritus Associated with Allergic Dermatitis
A masked, placebo-controlled field study was conducted at 15 veterinary clinics in the US, enrolling 306 client-owned dogs diagnosed with allergic dermatitis and having at least moderate pruritus. The allergic dermatitis was attributed to one or more of the following conditions: atopic dermatitis, contact dermatitis, flea allergy dermatitis, food hypersensitivity, or other. Dogs were randomized to once daily treatment with Zenrelia at 0.6 – 0.8 mg/kg or placebo, at a ratio of 2:1 respectively. Other medications that could affect the evaluation of effectiveness were not allowed during the study, such as corticosteroids, antihistamines, and cyclosporine. Treatment success for each dog was defined as a ≥ 50% reduction from baseline in owner-assessed pruritus scores on the Pruritus Visual Analog Scale (PVAS) on at least 5 out of the first 7 days of treatment. The proportion of dogs in the Zenrelia group that were treatment successes was greater than and significantly different compared to the placebo group on Day 7 (Table 4, below).
Table 4. Estimated Proportion of Dogs Achieving Treatment Success
* Based on back-transformed least squares means. † Placebo vs. Zenrelia p = 0.006 N = number of dogs Treatment Group
Estimated Proportion of Success*
Zenrelia (N = 193)
0.25
Placebo (N = 91)
0.08†
The Zenrelia group had a higher proportion of dogs with a ≥ 50% reduction from baseline in owner-assessed PVAS, compared to placebo, on Days 2 through 7.
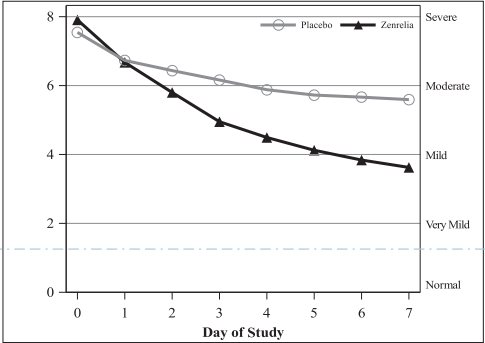
After Day 1, mean owner-assessed PVAS scores were lower in the Zenrelia group (Figure 1, below).
Figure 1. Mean owner-assessed PVAS Scores by Treatment for Days 0-7
Veterinarians used a dermatitis visual analog scale (DVAS) to assess each dog’s dermatitis. Veterinarian-assessed DVAS scores were lower for the Zenrelia group compared to the placebo group at all time points through Day 28.
-
TARGET ANIMAL SAFETY
Margin of Safety Study:
Zenrelia was administered to 40 (8 dogs per group) healthy, 11 to 12-month-old, fed Beagle dogs, once daily at 0X, 1X, 2X, 3X and 5X the maximum exposure dose of 0.8 mg/kg for 6 months. Control dogs were sham-dosed. Zenrelia-related clinical observations included a dose-dependent increase in the frequency and severity of interdigital furunculosis (cysts), with or without discharge on one or more paws, swollen and/or scabbing paws, and paw skin thickening and/or discoloration. Zenrelia-related hemogram findings included a dose-dependent minimal to moderate decrease in hematocrit (HCT), hemoglobin (HGB), and red blood cell (RBC) count without a corresponding increase in absolute reticulocyte count. Other Zenrelia-related findings included a minimal to mild decrease in mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentrations (MCHC), and eosinophil counts. Abnormal clinical pathology observations considered secondary to the interdigital furunculosis, included minimal to moderate increases in fibrinogen concentrations, total protein, C-reactive protein, and globulins, and decreases in albumin, albumin/globulin ratio and calcium levels. There were no Zenrelia-related effects on lymphocytes, monocytes, and basophils. Two dogs in the 5X group had minimally lower myeloid:erythroid ratios consistent with a physiological bone marrow response to the lower red blood cell mass despite no apparent effect on absolute reticulocytes. Zenrelia-related pathology changes included decreased prostate gland weights in the 5X group males and interdigital papillomas and/or dermatitis/furunculosis, predominantly in the 5X group. One dog in the 5X group had enlarged and mildly reactive draining lymph nodes associated with interdigital furunculosis. One dog in the 3X group had a papilloma on each paw with fragments of Demodex canis, and a follicular cyst within the markedly inflamed dermis.
Vaccine Response Study:
Zenrelia was administered to 16 (8 dogs per group) healthy, 10-month-old, vaccine naïve Beagle dogs, once daily at 0 and 3X the maximum exposure dose of 0.8 mg/kg for 89 days. Control dogs were placebo dosed. An 84-day recovery period followed in which no drug was administered. Dogs were administered a multivalent modified live virus (MLV) vaccine, including canine adenovirus type-2 (CAV-2), canine parvovirus (CPV), and canine distemper virus (CDV), on Days 28 and 60 and a killed rabies virus (RV) vaccine on Day 60. The primary endpoint was achievement on Day 88 of predefined serum titer thresholds considered adequate for protective immunity. Serum titers were also evaluated 27 days (RV only) and 83 days after discontinuing drug administration (Days 116 and 172, respectively).
On Day 52, one dog administered Zenrelia was euthanized due to lethargy, depression, poor body condition, and weakness. Necropsy revealed findings consistent with a colonic intussusception, potentially related to a clinical Cystoisospora canis infection secondary to Zenrelia-induced immunosuppression. On Day 54, another dog administered Zenrelia was euthanized due to lethargy, depression, poor body condition, and weakness. Histopathology evaluation revealed marked necrotizing hepatitis and pancreatitis and evidence of systemic endotoxemia. Prominent intranuclear inclusion bodies were found in the liver and pancreas, consistent with adenoviral infection. The adenoviral hepatitis and pancreatitis were concluded to be vaccine-induced, secondary to Zenrelia-induced immunosuppression.
Clinical signs observed in Zenrelia-treated dogs included poor body condition, pale mucous membranes, lethargy, diarrhea, vomiting, weight loss, decreased appetite, and depression likely due to a clinical C. canis infection secondary to Zenrelia-induced immunosuppression in seven of the eight dogs. No dogs in the control group were diagnosed with a C. canis infection. One dog in the control group had diarrhea. Zenrelia-related clinical observations also included interdigital cysts, lameness, and thickening and crusting of the ear margins. Clinical pathology findings in Zenrelia-treated dogs included decreases in hematocrit, hemoglobin, and red blood cell counts with a corresponding increase in absolute reticulocyte count, and decreases in total serum protein, albumin, and globulins, likely due to the C. canis infection.
On Day 88, all eight control dogs demonstrated an adequate immune response to CAV-2, CPV, CDV, and RV vaccination and all six remaining dogs receiving Zenrelia demonstrated an adequate immune response to CAV-2 and CPV vaccination. Four (of six) Zenrelia-treated dogs failed to demonstrate an adequate immune response to RV vaccination, and one (of six) Zenrelia-treated dogs failed to demonstrate an adequate immune response to CDV vaccination on Day 88.
During the recovery period, on Day 116 (27 days after discontinuing treatment), one (of eight) control dogs and one (of six) Zenrelia-treated dogs failed to demonstrate an adequate immune response to RV vaccination. On Day 172 (83 days after discontinuing treatment), all eight control dogs demonstrated an adequate immune response to CAV-2, CPV, and CDV vaccinations, all six remaining Zenrelia-treated dogs demonstrated an adequate immune response to CAV-2 and CPV vaccination, one (of six) Zenrelia-treated dogs failed to demonstrate an adequate immune response to CDV vaccination, and one (of eight) control dogs and three (of six) Zenrelia-treated dogs failed to demonstrate an adequate immune response to RV vaccination.
Potential recovery from drug-induced immunosuppression 27 days after discontinuing Zenrelia is evidenced by the observed adequate immune response to RV vaccination on Day 116 in three of the four dogs in the Zenrelia group that failed to achieve an adequate RV titer while receiving Zenrelia on Day 88. However, one of these four dogs did not achieve adequate RV titers after Zenrelia was discontinued for 83 days. A 3-month washout period prior to vaccination is supported by the 2024 World Small Animal Veterinary Association and 2023 Center for Disease Control and Prevention vaccination guidelines. The 28-day time period to withhold Zenrelia after vaccination is based on published and unpublished data evaluating the duration of MLV vaccine virus shedding.
Pilot Margin of Safety Study:
A non-final formulation of ilunocitinib (oral suspension) was administered via gavage to 32 (8 dogs per group) healthy, 9-month-old, Beagle dogs, once daily at 0X, 1X, 3X, and 4.5X the maximum exposure dose of 0.8 mg/kg through Day 64. Due to serious adverse reactions in the 3X and 4.5X groups, on Day 65, the 4.5X group was decreased to 2X through Day 185. Control dogs were sham dosed. One dog in the 4.5X group and two dogs in the 3X group were prematurely euthanized (on Days 52, 57, and 134, respectively) due to an acute onset of lethargy, labored breathing, fever, tremors, and pale gums, starting within four hours of dosing via oral gavage. Microscopic pathology findings in the three dogs included necrotizing hemorrhagic pneumonia, considered secondary to ilunocitinib-induced immunosuppression and gavage administration. Two of these three dogs developed severe leukopenia and neutropenia and one dog had severe weight loss in the two weeks prior to euthanasia. Twelve dogs administered ilunocitinib, including the three dogs prematurely euthanized, had a decrease in at least one RBC parameter (HCT, HGB, or RBC count), without a corresponding increase in absolute reticulocyte count, at one or more timepoints during the study. Additional ilunocitinib-related microscopic pathology changes included minimal to mild increased erythropoiesis and pigment in the spleen, minimal to mild pigmented macrophages in the liver, and minimal adipocyte accumulation in the bone marrow.
- STORAGE CONDITIONS
-
HOW SUPPLIED
Zenrelia (ilunocitinib tablets) is available in scored tablets in four strengths: 4.8 mg, 6.4 mg, 8.5 mg, and 15 mg. Each tablet strength is available in 10 and 30 count blister packages and 90 count bottles.
Manufactured for Elanco US Inc. Greefield, IN 46140
Approved by FDA under NADA # 141-585
Zenrelia, Elanco and the diagonal bar logo are trademarks
of Elanco or its affiliates. © 2024 Elanco or its affiliatesJuly 2024
PA104098XElanco™
-
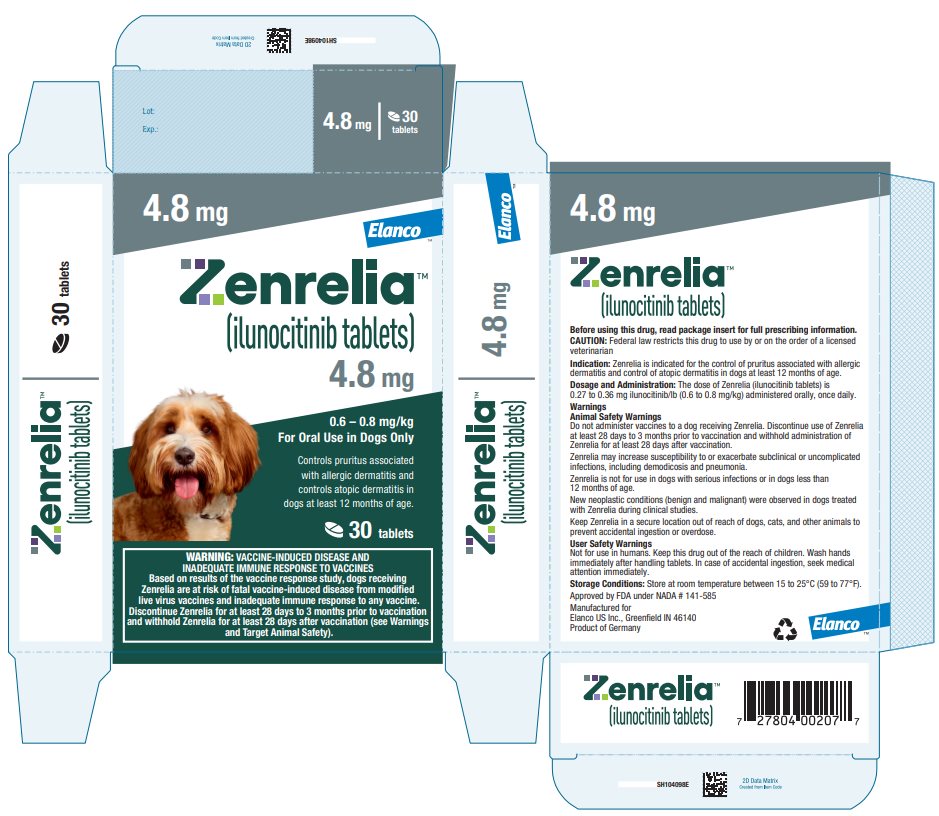
Principal Display Panel - Zenrelia 4.8 mg
Elanco™
Zenrelia™
(ilunocitinib tablets)
4.8 mg
0.6 – 0.8 mg/kg
For Oral Use in Dogs OnlyControls pruritus associated
with allergic dermatitis and
controls atopic dermatitis in
dogs at least 12 months of age.30 tablets
Elanco™
Zenrelia™
(ilunocitinib tablets)
4.8 mg
0.6 – 0.8 mg/kg
For Oral Use in Dogs OnlyControls pruritus associated
with allergic dermatitis and
controls atopic dermatitis in
dogs at least 12 months of age.90 tablets
-
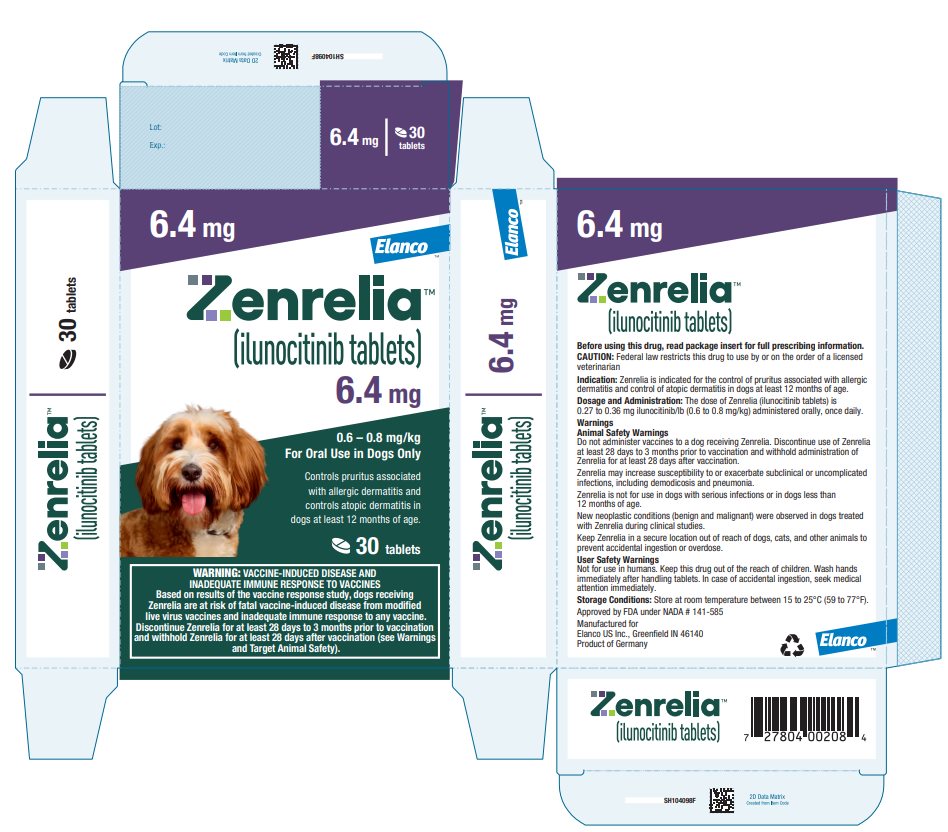
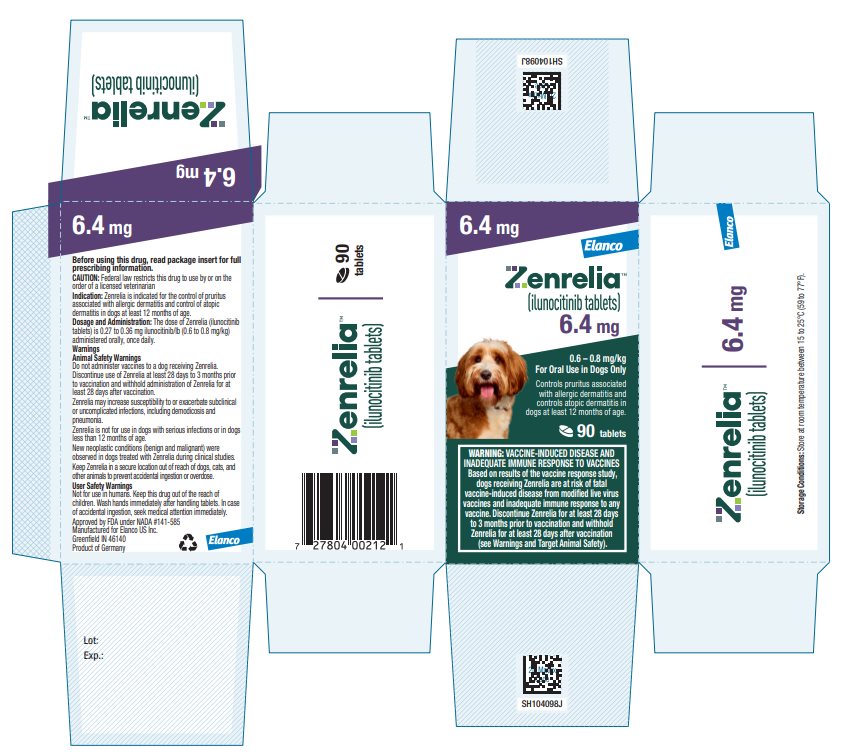
Principal Display Panel - Zenrelia 6.4 mg
Elanco™
Zenrelia™
(ilunocitinib tablets)
6.4 mg
0.6 – 0.8 mg/kg
For Oral Use in Dogs OnlyControls pruritus associated
with allergic dermatitis and
controls atopic dermatitis in
dogs at least 12 months of age.30 tablets
Elanco™
Zenrelia™
(ilunocitinib tablets)
6.4 mg
0.6 – 0.8 mg/kg
For Oral Use in Dogs OnlyControls pruritus associated
with allergic dermatitis and
controls atopic dermatitis in
dogs at least 12 months of age.90 tablets
-
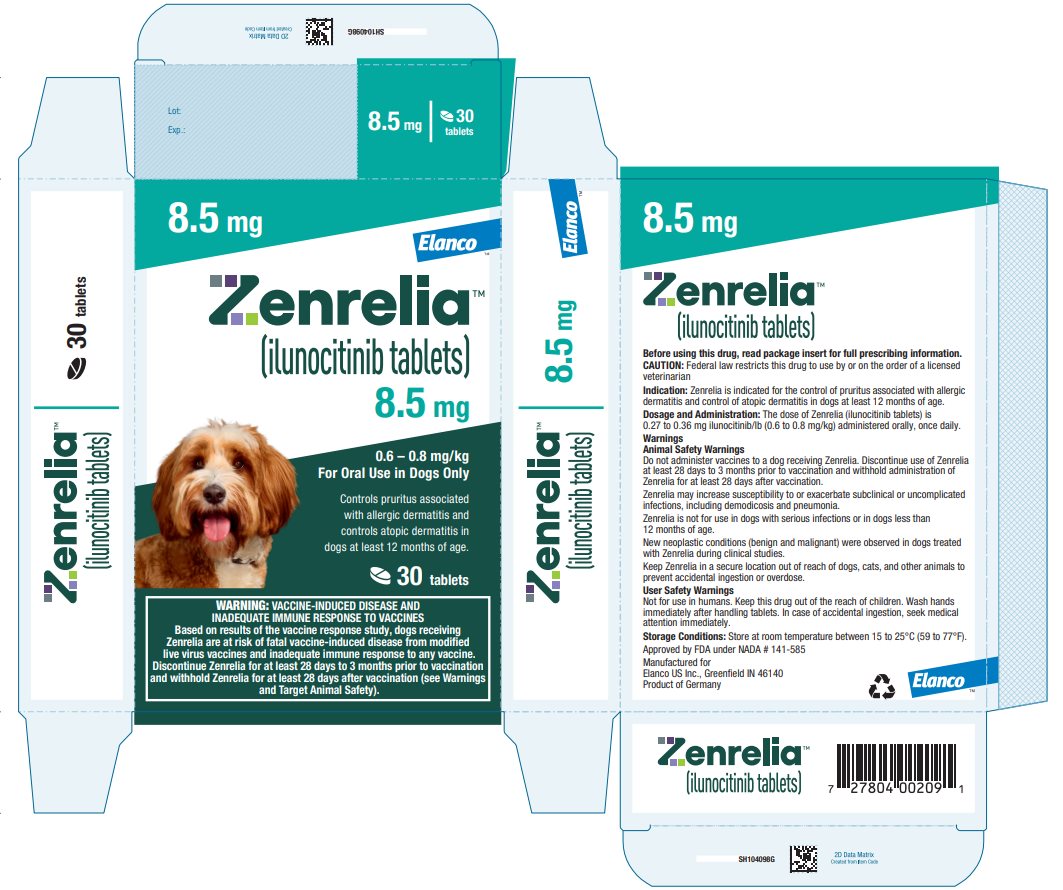
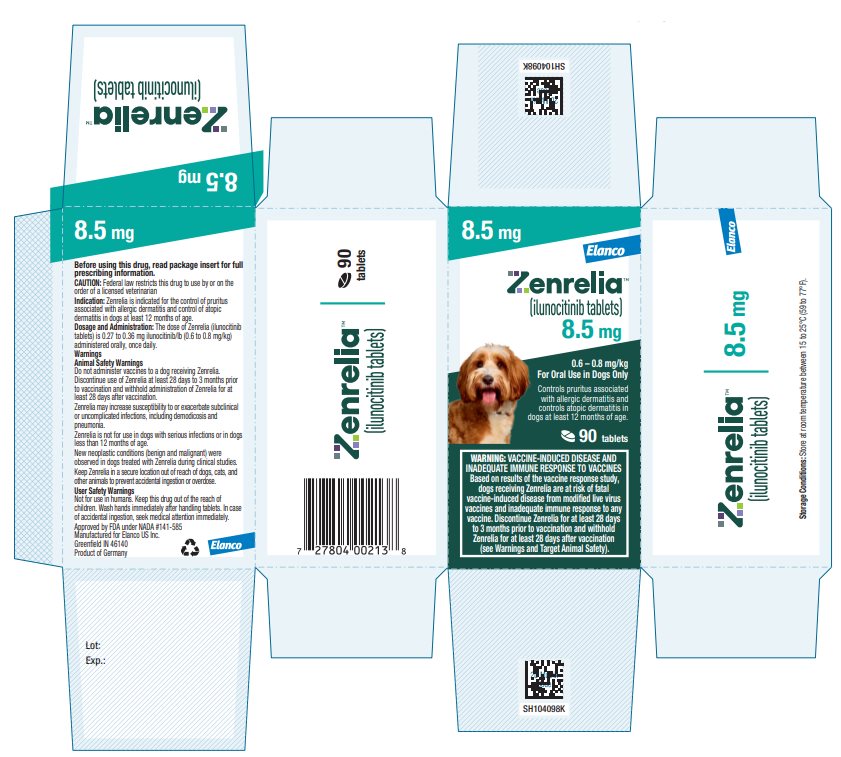
Principal Display Panel - Zenrelia 8.5 mg
Elanco™
Zenrelia™
(ilunocitinib tablets)
8.5 mg
0.6 – 0.8 mg/kg
For Oral Use in Dogs OnlyControls pruritus associated
with allergic dermatitis and
controls atopic dermatitis in
dogs at least 12 months of age.30 tablets
Elanco™
Zenrelia™
(ilunocitinib tablets)
8.5 mg
0.6 – 0.8 mg/kg
For Oral Use in Dogs OnlyControls pruritus associated
with allergic dermatitis and
controls atopic dermatitis in
dogs at least 12 months of age.90 tablets
-
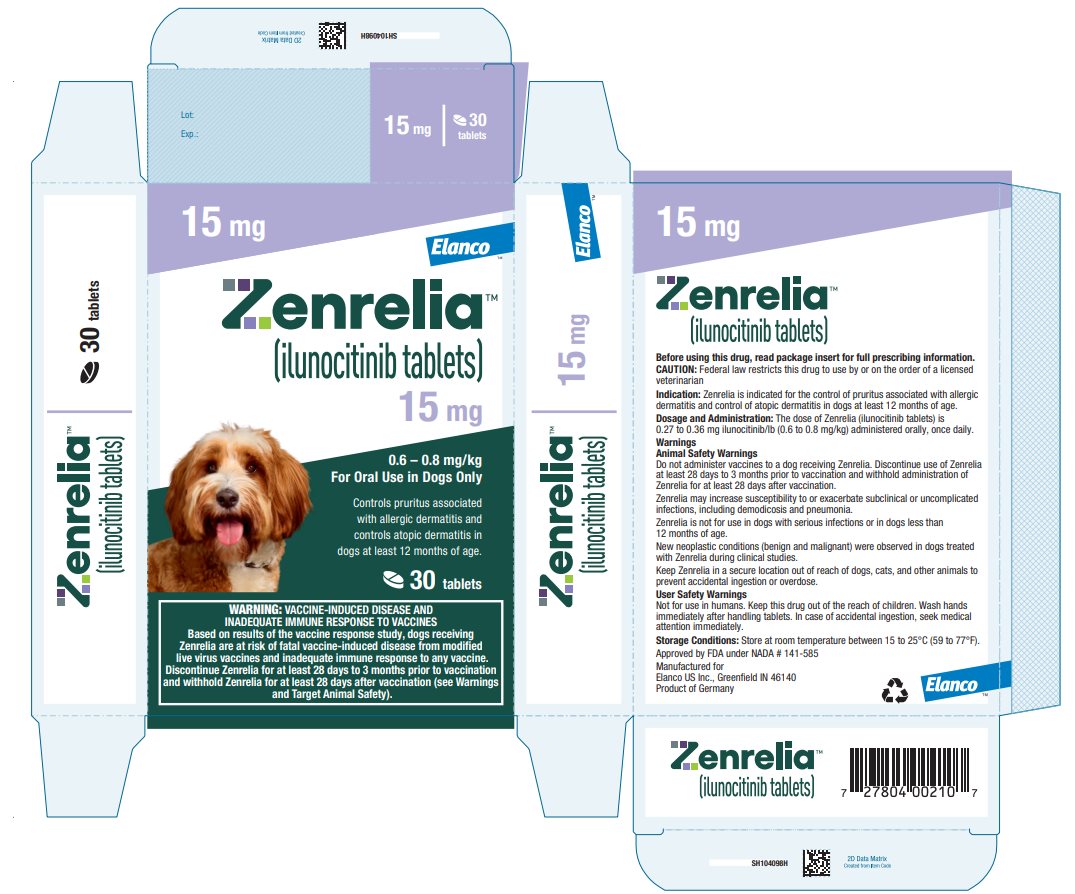
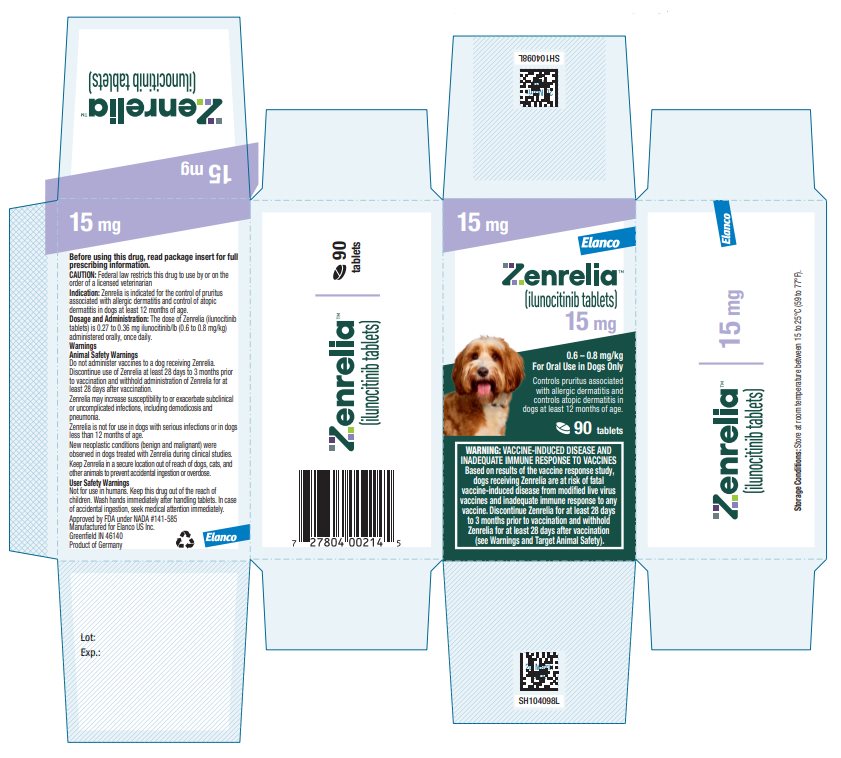
Principal Display Panel - Zenrelia 15 mg
Elanco™
Zenrelia™
(ilunocitinib tablets)
15 mg
0.6 – 0.8 mg/kg
For Oral Use in Dogs OnlyControls pruritus associated
with allergic dermatitis and
controls atopic dermatitis in
dogs at least 12 months of age.30 tablets
Elanco™
Zenrelia™
(ilunocitinib tablets)
15 mg
0.6 – 0.8 mg/kg
For Oral Use in Dogs OnlyControls pruritus associated
with allergic dermatitis and
controls atopic dermatitis in
dogs at least 12 months of age.90 tablets
-
INGREDIENTS AND APPEARANCE
ZENRELIA
ilunocitinib tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:58198-5536 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ILUNOCITINIB (UNII: N3TB5AH8B9) (ILUNOCITINIB - UNII:N3TB5AH8B9) ILUNOCITINIB 4.8 mg Product Characteristics Color YELLOW Score 2 pieces Shape OVAL (Oblong) Size 11mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58198-5536-1 3 in 1 CARTON 1 10 in 1 BLISTER PACK 2 NDC:58198-5536-2 6 in 1 BOX 2 3 in 1 CARTON 2 10 in 1 BLISTER PACK 3 NDC:58198-5536-3 1 in 1 CARTON 3 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141585 09/19/2024 ZENRELIA
ilunocitinib tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:58198-5537 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ILUNOCITINIB (UNII: N3TB5AH8B9) (ILUNOCITINIB - UNII:N3TB5AH8B9) ILUNOCITINIB 6.4 mg Product Characteristics Color YELLOW Score 2 pieces Shape OVAL (Oblong) Size 12mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58198-5537-1 3 in 1 CARTON 1 10 in 1 BLISTER PACK 2 NDC:58198-5537-2 6 in 1 BOX 2 3 in 1 CARTON 2 10 in 1 BLISTER PACK 3 NDC:58198-5537-3 1 in 1 CARTON 3 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141585 09/19/2024 ZENRELIA
ilunocitinib tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:58198-5538 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ILUNOCITINIB (UNII: N3TB5AH8B9) (ILUNOCITINIB - UNII:N3TB5AH8B9) ILUNOCITINIB 8.5 mg Product Characteristics Color YELLOW Score 2 pieces Shape OVAL (Oblong) Size 13mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58198-5538-1 3 in 1 CARTON 1 10 in 1 BLISTER PACK 2 NDC:58198-5538-2 6 in 1 BOX 2 3 in 1 CARTON 2 10 in 1 BLISTER PACK 3 NDC:58198-5538-3 1 in 1 CARTON 3 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141585 09/19/2024 ZENRELIA
ilunocitinib tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:58198-5539 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ILUNOCITINIB (UNII: N3TB5AH8B9) (ILUNOCITINIB - UNII:N3TB5AH8B9) ILUNOCITINIB 15 mg Product Characteristics Color YELLOW Score 2 pieces Shape OVAL (Oblong) Size 16mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58198-5539-1 3 in 1 CARTON 1 10 in 1 BLISTER PACK 2 NDC:58198-5539-2 6 in 1 BOX 2 3 in 1 CARTON 2 10 in 1 BLISTER PACK 3 NDC:58198-5539-3 1 in 1 CARTON 3 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141585 09/19/2024 Labeler - Elanco US Inc. (966985624)