Label: SELECT OB- .beta.-carotene, vitamin a acetate, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, levomefolate calcium, cobalamin, iron, magnesium oxide, and zinc oxide tablet, chewable
-
Contains inactivated NDC Code(s)
NDC Code(s): 0642-0120-03, 0642-0120-90 - Packager: Everett Laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated July 21, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
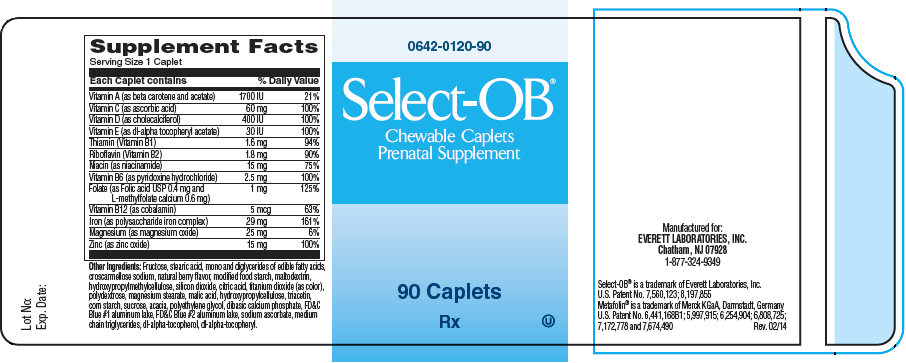
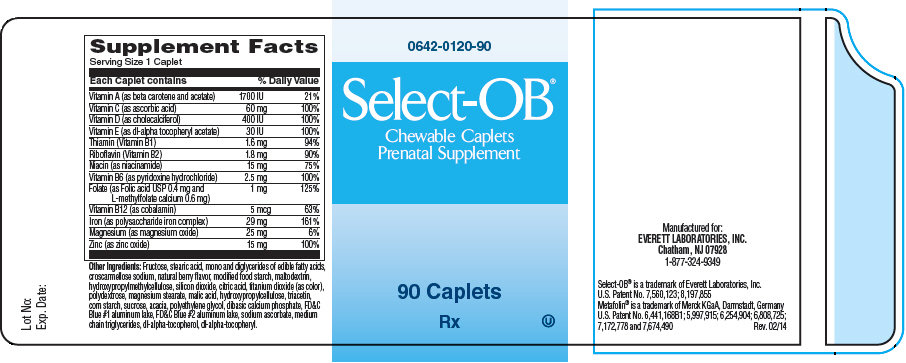
Supplement Facts Serving Size 1 Caplet Each Caplet contains % Daily Value Vitamin A (as beta carotene and acetate) 1700 IU 21% Vitamin C (as ascorbic acid) 60 mg 100% Vitamin D (as cholecalciferol) 400 IU 100% Vitamin E (as dl-alpha tocopheryl acetate) 30 IU 100% Thiamin (Vitamin B1) 1.6 mg 94% Riboflavin (Vitamin B2) 1.8 mg 90% Niacin (as niacinamide) 15 mg 75% Vitamin B6 (as pyridoxine hydrochloride) 2.5 mg 100% Folate (as Folic acid USP 0.4 mg and
L-methylfolate calcium 0.6 mg)1 mg 125% Vitamin B12 (as cobalamin) 5 mcg 63% Iron (as polysaccharide iron complex) 29 mg 161% Magnesium (as magnesium oxide) 25 mg 6% Zinc (as zinc oxide) 15 mg 100% Other Ingredients: Fructose, stearic acid, mono and diglycerides of edible fatty acids, croscarmellose sodium, natural berry flavor, modified food starch, maltodextrin, hydroxypropylmethylcellulose, silicon dioxide, citric acid, titanium dioxide (as color), polydextrose, magnesium stearate, malic acid, hydroxypropylcellulose, triacetin, corn starch, sucrose, acacia, polyethylene glycol, dibasic calcium phosphate, FD&C Blue #1 aluminum lake, FD&C Blue #2 aluminum lake, sodium ascorbate, medium chain triglycerides, dl-alpha-tocopherol, dl-alpha-tocopheryl.
-
CONTRAINDICATIONS
SELECT-OB® Chewable Caplets are contraindicated in patients with hypersensitivity to any of its components or color additives.
Folic acid is contraindicated in patients with untreated and uncomplicated pernicious anemia, and in those with anaphylactic sensitivity to folic acid.
Iron therapy is contraindicated in patients with hemochromatosis and patients with iron storage disease or the potential for iron storage disease due to chronic hemolytic anemia (e.g., inherited anomalies of hemoglobin structure or synthesis and/or red cell enzyme deficiencies, etc.), pyridoxine responsive anemia, or cirrhosis of the liver.
Cyanocobalamin is contraindicated in patients with sensitivity to cobalt or to cyanocobalamin (vitamin B12).
- BOXED WARNING (What is this?)
-
WARNINGS/PRECAUTIONS
Vitamin D supplementation should be used with caution in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones. High doses of vitamin D can lead to elevated levels of calcium that reside in the blood and soft tissues. Bone pain, high blood pressure, formation of kidney stones, renal failure, and increased risk of heart disease can occur. Prolonged use of iron salts may produce iron storage disease.
Folic acid, especially in doses above 0.1 mg daily, may obscure pernicious anemia, in that hematologic remission may occur while neurological manifestations remain progressive. The use of folic acid doses above 1 mg daily may precipitate or exacerbate the neurological damage of vitamin B12 deficiency.
Avoid overdosage. Keep out of the reach of children.
Drug Interactions
High doses of folic acid may result in decreased serum levels of the anticonvulsant drugs; carbamazepine, fosphenytoin, phenytoin, phenobarbital, valproic acid. Folic acid may decrease a patient's response to methotrexate. Vitamin D supplementation should not be given with large amounts of calcium in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones. Zinc can inhibit the absorption of certain antibiotics; take at least 2 hours apart to minimize interactions. Consult appropriate references for additional specific vitamin-drug interactions.
-
ADVERSE REACTIONS
Adverse reactions have been reported with specific vitamins and minerals, but generally at doses substantially higher than those in SELECT-OB® Chewable Caplets. However, allergic and idiosyncratic reactions are possible at any dose. Reported adverse events include skin ailments, gastrointestinal complaints, glucose abnormalities, and visual problems.
- HOW SUPPLIED
- USAGE
- DIRECTIONS FOR USE
- SPL UNCLASSIFIED SECTION
- STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 90 Caplet Bottle Label
-
INGREDIENTS AND APPEARANCE
SELECT OB
.beta.-carotene, vitamin a acetate, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, levomefolate calcium, cobalamin, iron, magnesium oxide, and zinc oxide tablet, chewableProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0642-0120 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength .BETA.-CAROTENE (UNII: 01YAE03M7J) (.BETA.-CAROTENE - UNII:01YAE03M7J) .BETA.-CAROTENE 600 [iU] VITAMIN A ACETATE (UNII: 3LE3D9D6OY) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 1100 [iU] ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 60 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 400 [iU] .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 30 [iU] THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 1.6 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 1.8 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 15 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 2.5 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 0.4 mg LEVOMEFOLATE CALCIUM (UNII: A9R10K3F2F) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLATE CALCIUM 0.6 mg COBALAMIN (UNII: 8406EY2OQA) (COBALAMIN - UNII:8406EY2OQA) COBALAMIN 5 ug IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 29 mg MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM OXIDE 25 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 15 mg Inactive Ingredients Ingredient Name Strength FRUCTOSE (UNII: 6YSS42VSEV) STEARIC ACID (UNII: 4ELV7Z65AP) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MALTODEXTRIN (UNII: 7CVR7L4A2D) HYPROMELLOSES (UNII: 3NXW29V3WO) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYDEXTROSE (UNII: VH2XOU12IE) MAGNESIUM STEARATE (UNII: 70097M6I30) MALIC ACID (UNII: 817L1N4CKP) HYDROXYPROPYL CELLULOSE (TYPE H) (UNII: RFW2ET671P) TRIACETIN (UNII: XHX3C3X673) STARCH, CORN (UNII: O8232NY3SJ) SUCROSE (UNII: C151H8M554) ACACIA (UNII: 5C5403N26O) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) SODIUM ASCORBATE (UNII: S033EH8359) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL, DL- (UNII: 7QWA1RIO01) Product Characteristics Color BLUE Score no score Shape OVAL Size 19mm Flavor BERRY Imprint Code EV0120 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0642-0120-90 90 in 1 BOTTLE 2 NDC:0642-0120-03 3 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 07/14/2014 Labeler - Everett Laboratories, Inc. (071170534)