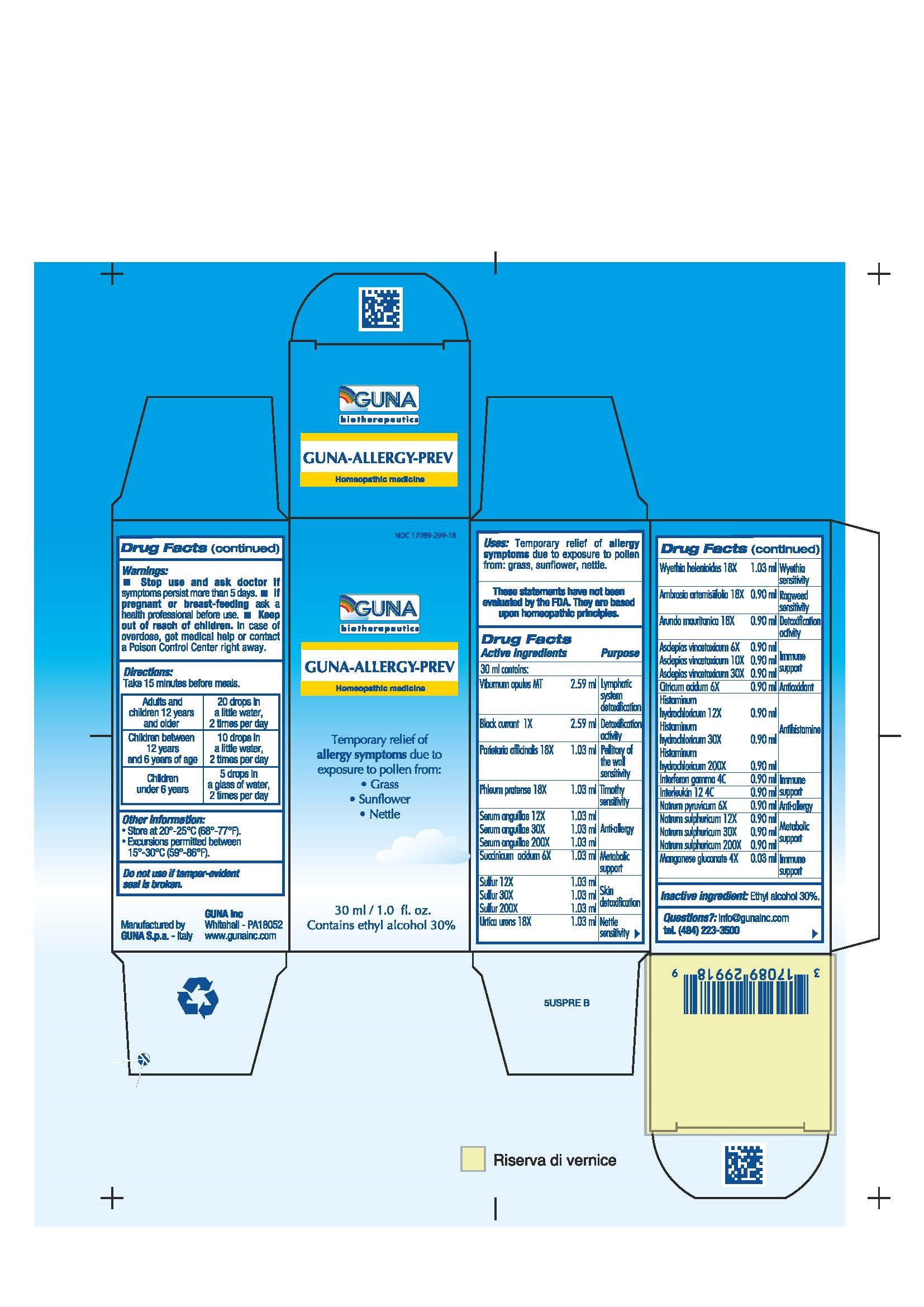
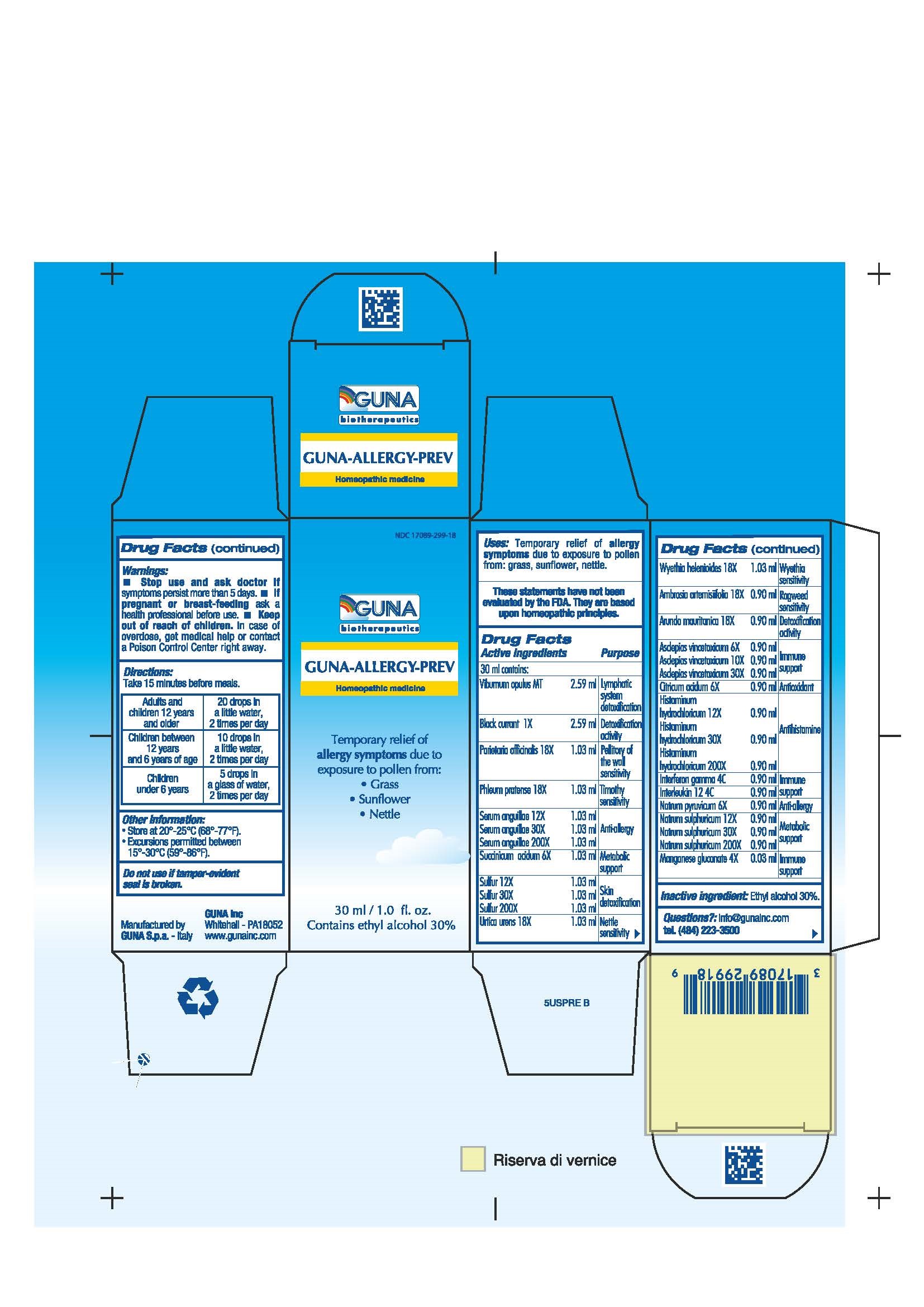
Label: GUNA-ALLERGY-PREV- ambrosia artemisiifolia - anguilla rostrata blood serum - arundo pliniana root - black currant - chelidonium majus - citric acid monohydrate - histamine dihydrochloride - human interleukin 12 - interferon gamma-1b - manganese gluconate - parietaria officinalis - phleum pratense - sodium pyruvate - sodium sulfate - succinic acid - sulfur - urtica urens - viburnum opulus root - wyethia helenioides root - solution/ drops
- NDC Code(s): 17089-299-18
- Packager: Guna spa
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 21, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTS/PURPOSE
AMBROSIA ARTEMISIIFOLIA 18X RAGWEED SENSITIVITY
ARUNDO MAURITANICA 18X DETOXIFICATION ACTIVITY
ASCLEPIAS VINCETOXICUM 6X, 10X, 30X IMMUNE SUPPORT
BLACK CURRANT 1X DETOXIFICATION ACTIVITY
CITRICUM ACIDUM 6X ANTIOXIDANT
HISTAMINUM HYDROCHLORICUM 12X, 30X, 200X ANTIHISTAMINE
INTERFERON GAMMA 4C IMMUNE SUPPORT
INTERLEUKIN 12 4C IMMUNE SUPPORT
MANGANESE GLUCONATE 4X IMMUNE SUPPORT
NATRUM PYRUVICUM 6X ANTI-ALLERGY
NATRUM SULF 12X, 30X, 200X METABOLIC SUPPORT
PARIETARIA OFFICINALIS 18X PELLITORY OF THE WALL SENSITIVITY
PHLEUM PRATENSE 18X TIMOTHY SENSITIVITY
SERUM ANGUILLAE 12X, 30X, 200X ANTI-ALLERGY
SUCCINICUM ACIDUM 6X METABOLIC SUPPORT
SULFUR 12X, 30X, 200X SKIN DETOXIFICATION
URTICA URENS 18X NETTLE SENSITIVITY
VIBURNUM OPULUS T LYMPHATIC SYSTEM DETOXIFICATION
WYETHIA HELENIOIDES 18X WYETHIA SENSITIVITY
- USES
- WARNINGS
- PREGNANCY
- WARNINGS
-
DIRECTIONS
For maximum symptom relief start before the anticipated allergen exposure.
Adults and children 12 years and older 20 drops in a little water, 2 times per day
Children between 12 years and 6 years of age 10 drops in a little water, 2 times per day
Children under 6 years 5 drops in a glass of water, 2 times per day - QUESTIONS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-ALLERGY-PREV
ambrosia artemisiifolia - anguilla rostrata blood serum - arundo pliniana root - black currant - chelidonium majus - citric acid monohydrate - histamine dihydrochloride - human interleukin 12 - interferon gamma-1b - manganese gluconate - parietaria officinalis - phleum pratense - sodium pyruvate - sodium sulfate - succinic acid - sulfur - urtica urens - viburnum opulus root - wyethia helenioides root - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-299 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMBROSIA ARTEMISIIFOLIA (UNII: 9W34L2CQ9A) (AMBROSIA ARTEMISIIFOLIA - UNII:9W34L2CQ9A) AMBROSIA ARTEMISIIFOLIA 18 [hp_X] in 30 mL ARUNDO PLINIANA ROOT (UNII: ZXE7LB03WC) (ARUNDO PLINIANA ROOT - UNII:ZXE7LB03WC) ARUNDO PLINIANA ROOT 18 [hp_X] in 30 mL ASCLEPIAS CURASSAVICA (UNII: JSZ93E47EP) (ASCLEPIAS CURASSAVICA - UNII:JSZ93E47EP) ASCLEPIAS CURASSAVICA 6 [hp_X] in 30 mL BLACK CURRANT (UNII: 9755T40D11) (BLACK CURRANT - UNII:9755T40D11) BLACK CURRANT 1 [hp_X] in 30 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 6 [hp_X] in 30 mL HISTAMINE DIHYDROCHLORIDE (UNII: 3POA0Q644U) (HISTAMINE - UNII:820484N8I3) HISTAMINE DIHYDROCHLORIDE 30 [hp_X] in 30 mL INTERFERON GAMMA-1B (UNII: 21K6M2I7AG) (INTERFERON GAMMA-1B - UNII:21K6M2I7AG) INTERFERON GAMMA-1B 4 [hp_C] in 30 mL HUMAN INTERLEUKIN 12 (UNII: 7B590791ER) (HUMAN INTERLEUKIN 12 - UNII:7B590791ER) HUMAN INTERLEUKIN 12 4 [hp_C] in 30 mL MANGANESE GLUCONATE (UNII: 9YY2F980SV) (MANGANESE CATION (2+) - UNII:H6EP7W5457) MANGANESE GLUCONATE 4 [hp_X] in 30 mL SODIUM PYRUVATE (UNII: POD38AIF08) (PYRUVIC ACID - UNII:8558G7RUTR) SODIUM PYRUVATE 6 [hp_X] in 30 mL SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM SULFATE 200 [hp_X] in 30 mL PARIETARIA OFFICINALIS (UNII: 2C839WQ03N) (PARIETARIA OFFICINALIS - UNII:2C839WQ03N) PARIETARIA OFFICINALIS 18 [hp_X] in 30 mL PHLEUM PRATENSE (UNII: S7PW24BX20) (PHLEUM PRATENSE - UNII:S7PW24BX20) PHLEUM PRATENSE 18 [hp_X] in 30 mL ANGUILLA ROSTRATA BLOOD SERUM (UNII: L7B16ESD1U) (ANGUILLA ROSTRATA BLOOD SERUM - UNII:L7B16ESD1U) ANGUILLA ROSTRATA BLOOD SERUM 200 [hp_X] in 30 mL SUCCINIC ACID (UNII: AB6MNQ6J6L) (SUCCINIC ACID - UNII:AB6MNQ6J6L) SUCCINIC ACID 6 [hp_X] in 30 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 30 [hp_X] in 30 mL URTICA URENS (UNII: IHN2NQ5OF9) (URTICA URENS - UNII:IHN2NQ5OF9) URTICA URENS 18 [hp_X] in 30 mL VIBURNUM OPULUS ROOT (UNII: T1UG6H6805) (VIBURNUM OPULUS ROOT - UNII:T1UG6H6805) VIBURNUM OPULUS ROOT 3 g in 30 mL WYETHIA HELENIOIDES ROOT (UNII: J10PD1AQ0N) (WYETHIA HELENIOIDES ROOT - UNII:J10PD1AQ0N) WYETHIA HELENIOIDES ROOT 18 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-299-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/23/2006 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-299)