Label: BUPIVACAINE HYDROCHLORIDE injection, solution
- NDC Code(s): 72603-279-01, 72603-279-25, 72603-287-01, 72603-287-25
- Packager: Northstar Rx LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BUPIVACAINE HYDROCHLORIDE INJECTION safely and effectively. See full prescribing information for BUPIVACAINE HYDROCHLORIDE ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: RISK OF CARDIAC ARREST WITH USE OF BUPIVACAINE HYDROCHLORIDE IN OBSTETRICAL ANESTHESIA
There have been reports of cardiac arrest with difficult resuscitation or death during use of bupivacaine hydrochloride for epidural anesthesia in obstetrical patients. In most cases, this has followed use of the 0.75% (7.5 mg per mL) concentration. Resuscitation has been difficult or impossible despite apparently adequate preparation and appropriate management. Cardiac arrest has occurred after convulsions resulting from systemic toxicity, presumably following unintentional intravascular injection. The 0.75% (7.5 mg per mL) concentration of bupivacaine hydrochloride is not recommended for obstetrical anesthesia and should be reserved for surgical procedures where a high degree of muscle relaxation and prolonged effect are necessary [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGEBupivacaine Hydrochloride Injection is indicated in adults for the production of local or regional anesthesia or analgesia for surgery and oral surgery procedures, diagnostic and therapeutic ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Information - Bupivacaine hydrochloride injection is not for intrathecal use. Avoid use of bupivacaine hydrochloride injection solutions containing ...
-
3 DOSAGE FORMS AND STRENGTHS
Bupivacaine Hydrochloride Injection, USP is a clear, colorless solution available as: 0.25% (125 mg per 50 mL) (2.5 mg per mL) in multiple dose vials. 0.5% (250 mg per 50 mL) (5 mg per mL) in ...
-
4 CONTRAINDICATIONS
Bupivacaine hydrochloride is contraindicated in: obstetrical paracervical block anesthesia. Its use in this technique has resulted in fetal bradycardia and death. intravenous regional anesthesia ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Cardiac Arrest with Use of Bupivacaine Hydrochloride in Obstetrical Anesthesia - There have been reports of cardiac arrest with difficult resuscitation or death during use of ...
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions have been reported and described in the Warnings and Precautions section of the labeling: Cardiac Arrest in Obstetrical Anesthesia [see ...
-
7 DRUG INTERACTIONS
7.1 Local Anesthetics - The toxic effects of local anesthetics are additive. If coadministration of other local anesthetics with bupivacaine hydrochloride cannot be avoided, monitor patients for ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Bupivacaine hydrochloride is contraindicated for obstetrical paracervical block anesthesia. Its use in this technique has resulted in fetal bradycardia and ...
-
10 OVERDOSAGE
Clinical Presentation - Acute emergencies from use of bupivacaine hydrochloride are generally related to high plasma levels encountered during therapeutic use or to unintended intrathecal ...
-
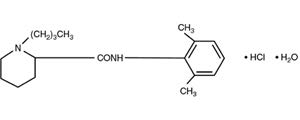
11 DESCRIPTIONBupivacaine Hydrochloride Injection, USP contains bupivacaine hydrochloride, an amide local anesthetic, as the active pharmaceutical ingredient. The route of administration for Bupivacaine ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Bupivacaine hydrochloride blocks the generation and the conduction of nerve impulses, presumably by increasing the threshold for electrical excitation in the nerve, by ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term studies in animals to evaluate the carcinogenic potential of bupivacaine hydrochloride have not been ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Bupivacaine Hydrochloride Injection, USP is a clear and colorless sterile isotonic solution, and is supplied as follows: NDCBupivacaine Hydrochloride Injection, USP 0.25% (2.5 mg per ...
-
17 PATIENT COUNSELING INFORMATION
Allergic-Type Reactions - Assess if the patient has had allergic-type reactions to amide-type local anesthetics or to other formulation ingredients, such as the antimicrobial preservative ...
-
PRINCIPAL DISPLAY PANEL – 0.25% Bupivacaine Hydrochloride Injection, USP 125 mg per 50 mL Vial LabelNDC 72603-279-01 - Rx Only - Bupivacaine Hydrochloride Injection, USP 0.25% 125 mg per 50 mL - (2.5 mg per mL) For INFILTRATION and NERVE BLOCK - 50 mL Multiple Dose Vial
-
PRINCIPAL DISPLAY PANEL – 0.25% Bupivacaine Hydrochloride Injection, USP 125 mg per 50 mL CartonNDC 72603-279-25 - Rx Only - Bupivacaine Hydrochloride Injection, USP 0.25% 125 mg per 50 mL - (2.5 mg per mL) For INFILTRATION and NERVE BLOCK - NOT FOR CAUDAL, EPIDURAL, OR SPINAL ...
-
PRINCIPAL DISPLAY PANEL – 0.5% Bupivacaine Hydrochloride Injection, USP 250 mg per 50 mL Vial LabelNDC 72603-287-01 - Rx Only - Bupivacaine Hydrochloride Injection, USP 0.5% 250 mg per 50 mL - (5 mg per mL) For NERVE BLOCK - 50 mL Multiple Dose Vial
-
PRINCIPAL DISPLAY PANEL – 0.5% Bupivacaine Hydrochloride Injection, USP 250 mg per 50 mL CartonNDC 72603-287-25 - Rx Only - Bupivacaine Hydrochloride Injection, USP 0.5% 250 mg per 50 mL - (5 mg per mL) For NERVE BLOCK - NOT FOR CAUDAL, EPIDURAL, OR SPINAL ANESTHESIA - 25 x 50 mL Multiple ...
-
INGREDIENTS AND APPEARANCEProduct Information