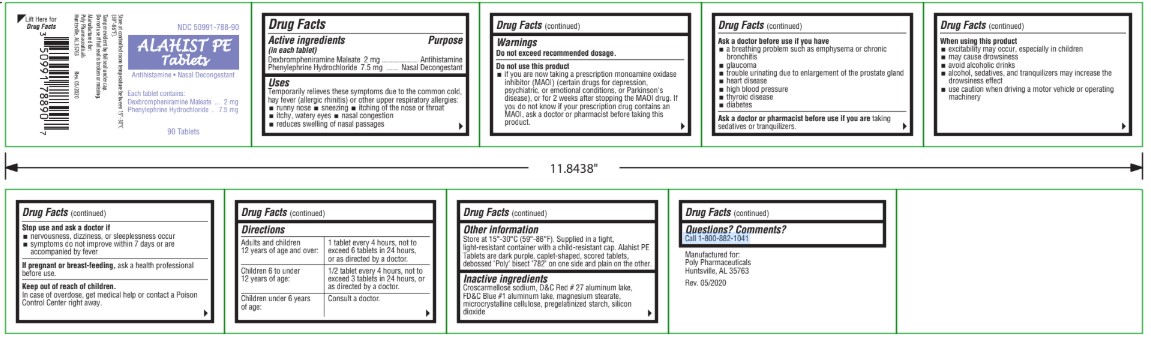
Label: ALAHIST PE- dexbrompheniramine maleate, phenylephrine hydrochloride tablet
- NDC Code(s): 50991-788-02, 50991-788-90
- Packager: Poly Pharmaceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
-
Warnings
Do not use this product
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to enlargement of the prostate gland
- heart disease
- high blood pressure
- thyroid disease
- diabetes
Ask a doctor or pharmacist before use if you are taking
sedatives or tranquilizers.When Using This product
- excitability may occur, especially in children
- may cause drowsiness
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase the drowsiness effect
- use caution when driving a motor vehicle or operating machinery
-
Directions
Adults and children 12 years of age and over: 1 tablet every 4 hours, not to exceed 6 tablets in 24 hours, or as directed by a doctor. Children 6 to under 12 years of age 1/2 tablet every 4 hours, not to exceed 3 tablets in 24 hours, or as directed by a doctor. Children under 6 years of age: Consult a doctor. - Other Information
- Inactive ingredients
- Questions? Comments?
- Alahist PE Label
-
INGREDIENTS AND APPEARANCE
ALAHIST PE
dexbrompheniramine maleate, phenylephrine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50991-788 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXBROMPHENIRAMINE MALEATE (UNII: BPA9UT29BS) (DEXBROMPHENIRAMINE - UNII:75T64B71RP) DEXBROMPHENIRAMINE MALEATE 2 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 7.5 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) STARCH, CORN (UNII: O8232NY3SJ) D&C RED NO. 27 (UNII: 2LRS185U6K) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color purple Score 2 pieces Shape CAPSULE Size 11mm Flavor Imprint Code Poly;782 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50991-788-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 08/24/2020 2 NDC:50991-788-02 12 in 1 BLISTER PACK; Type 0: Not a Combination Product 08/24/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/24/2020 Labeler - Poly Pharmaceuticals, Inc. (198449894)