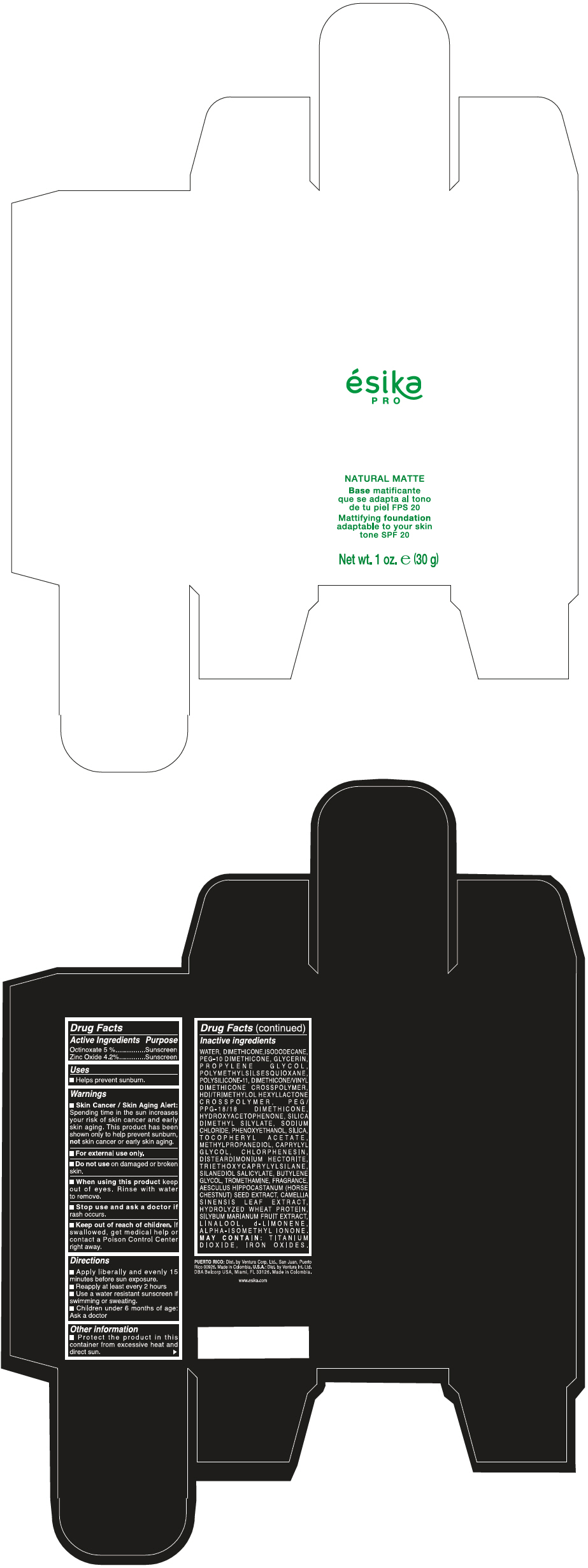
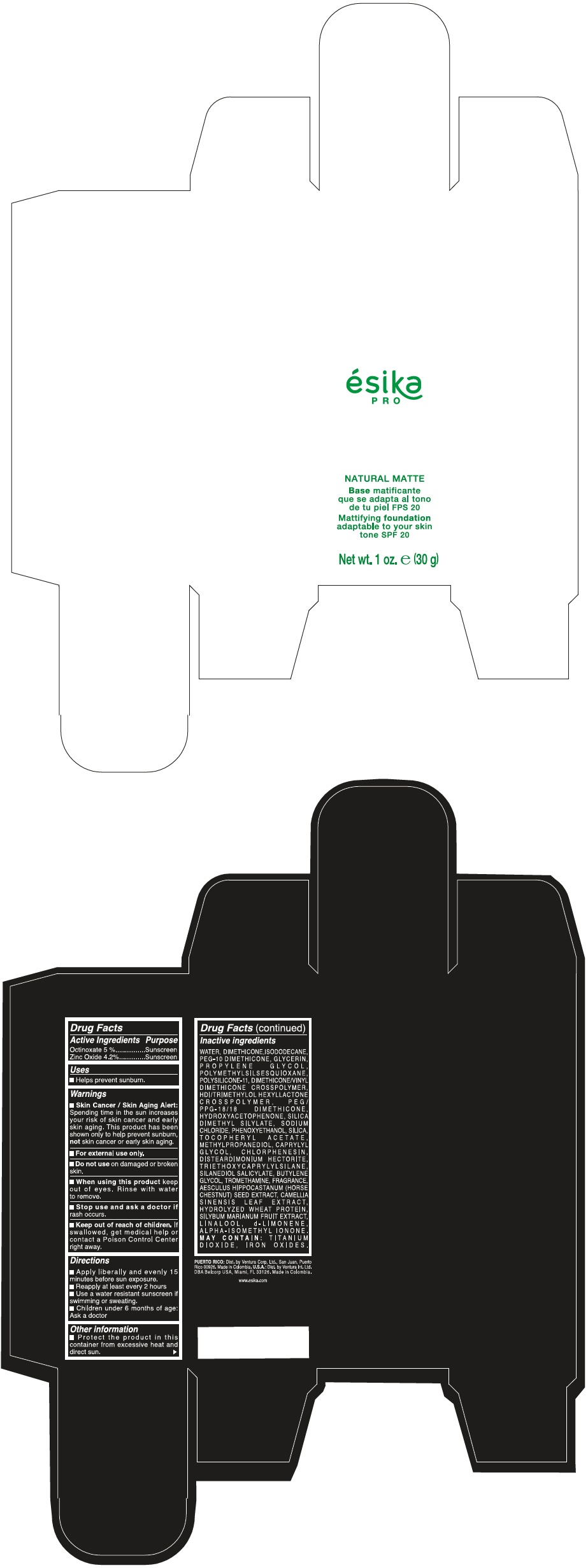
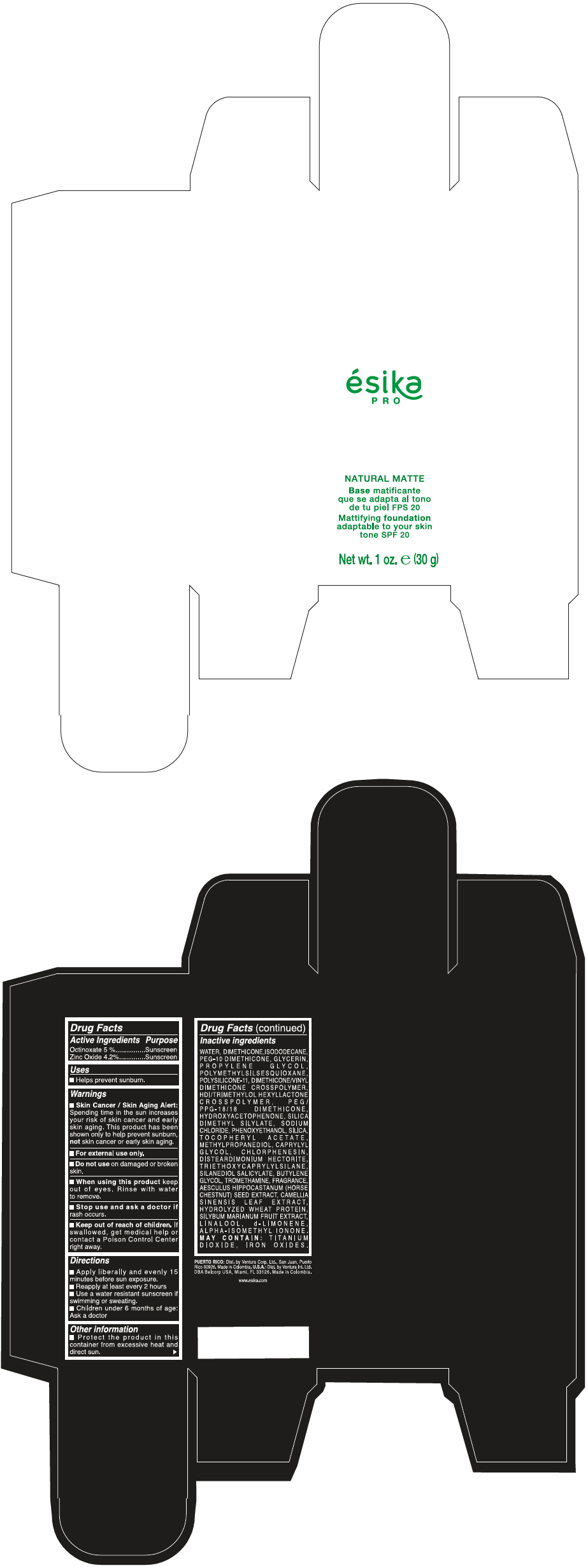
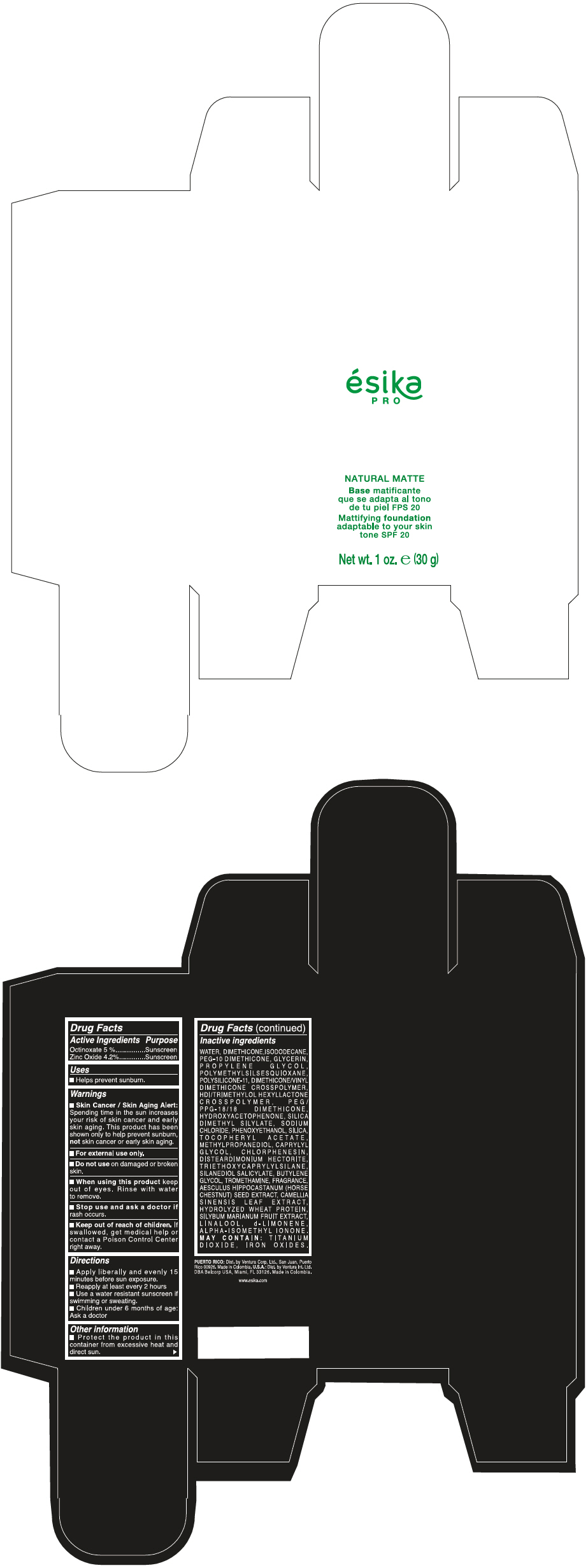
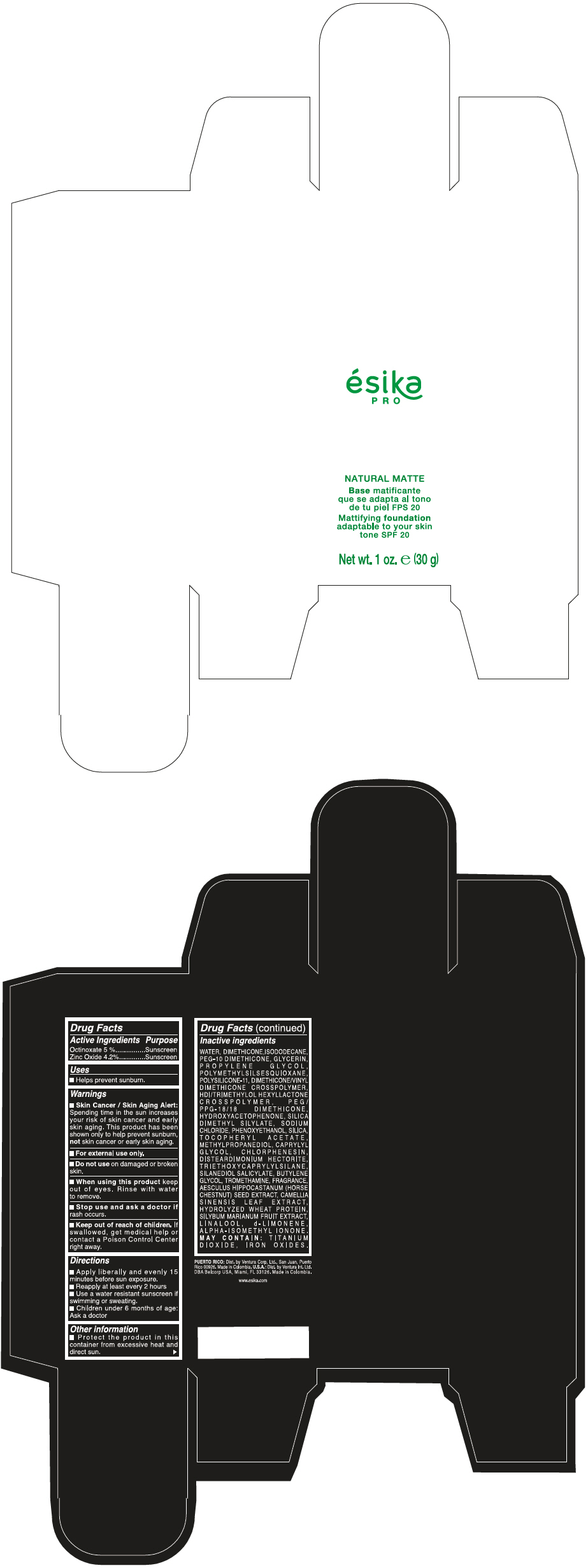
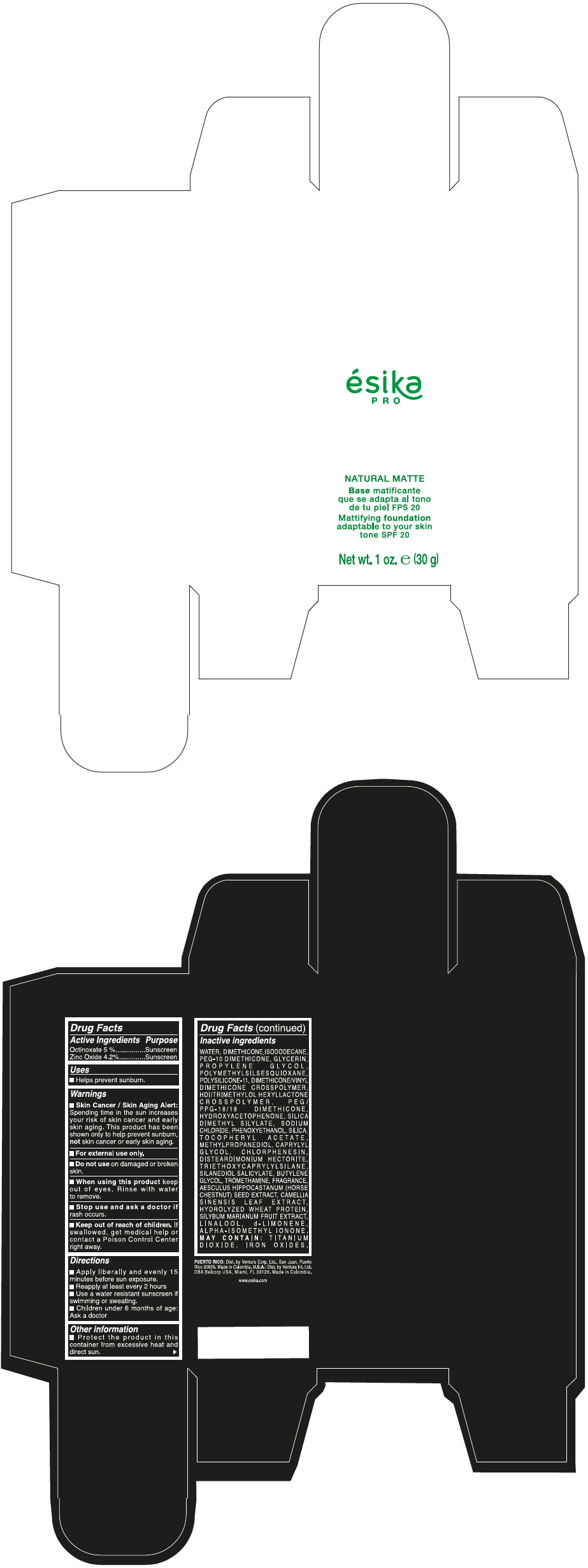
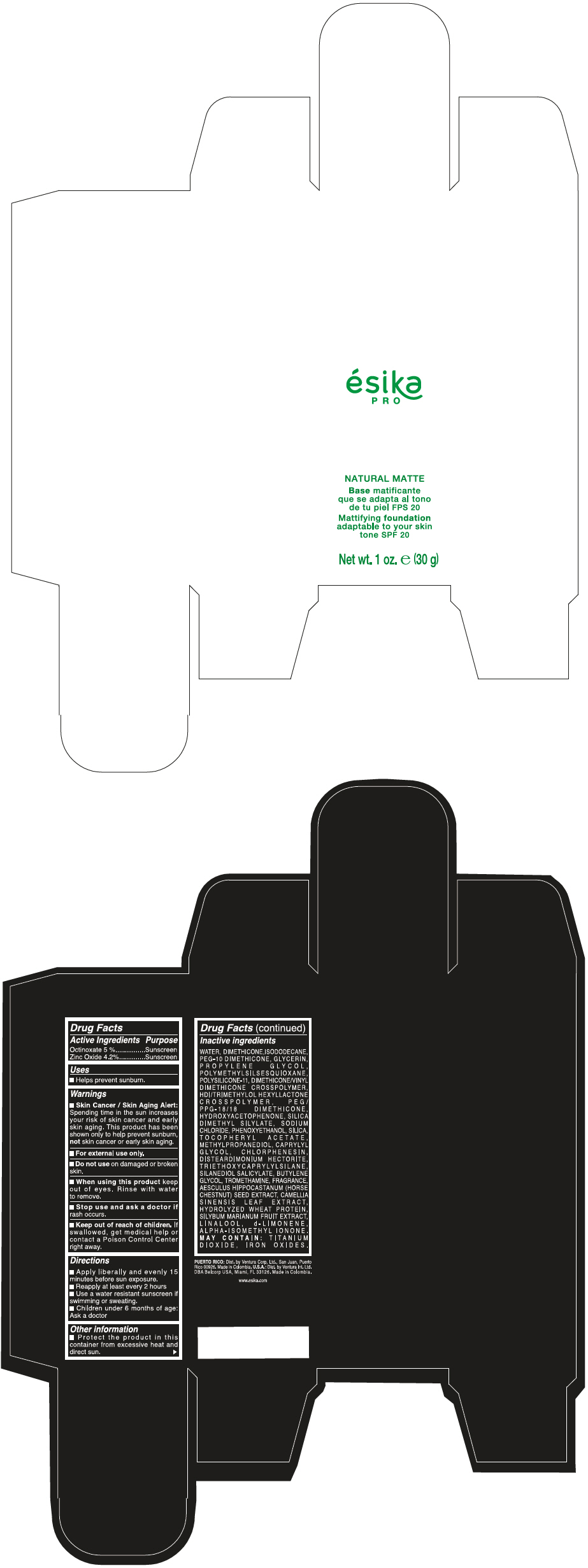
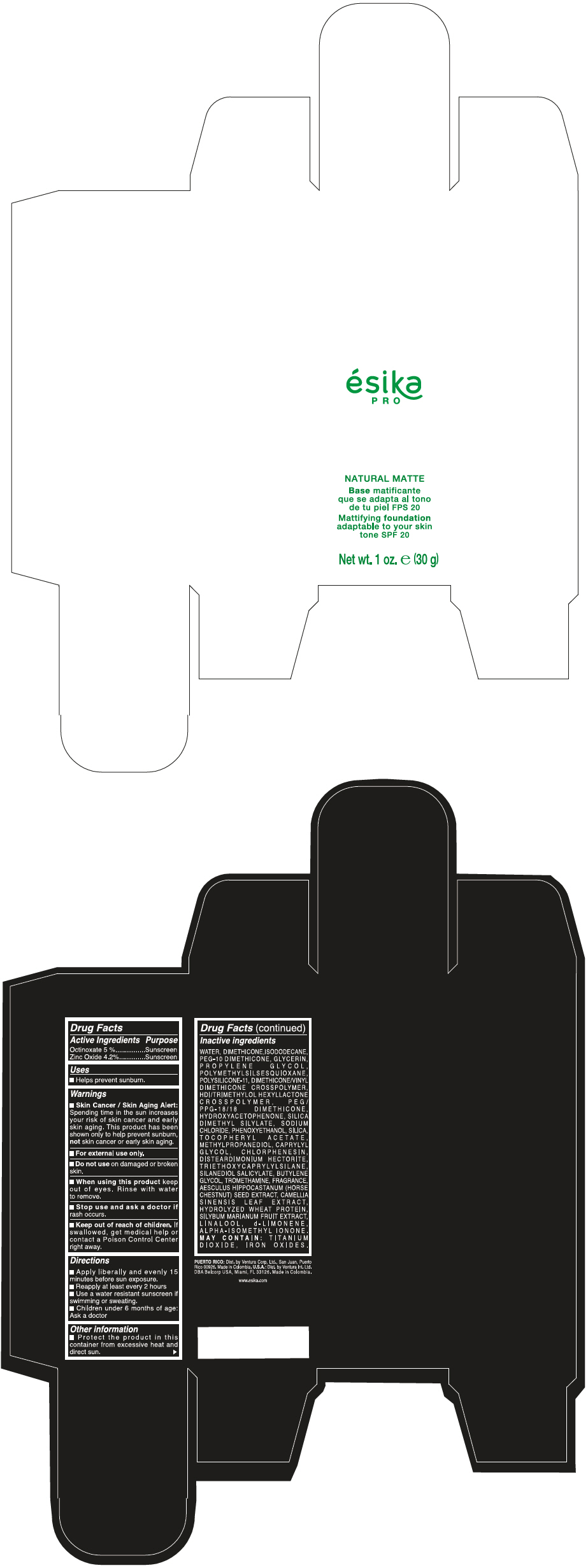
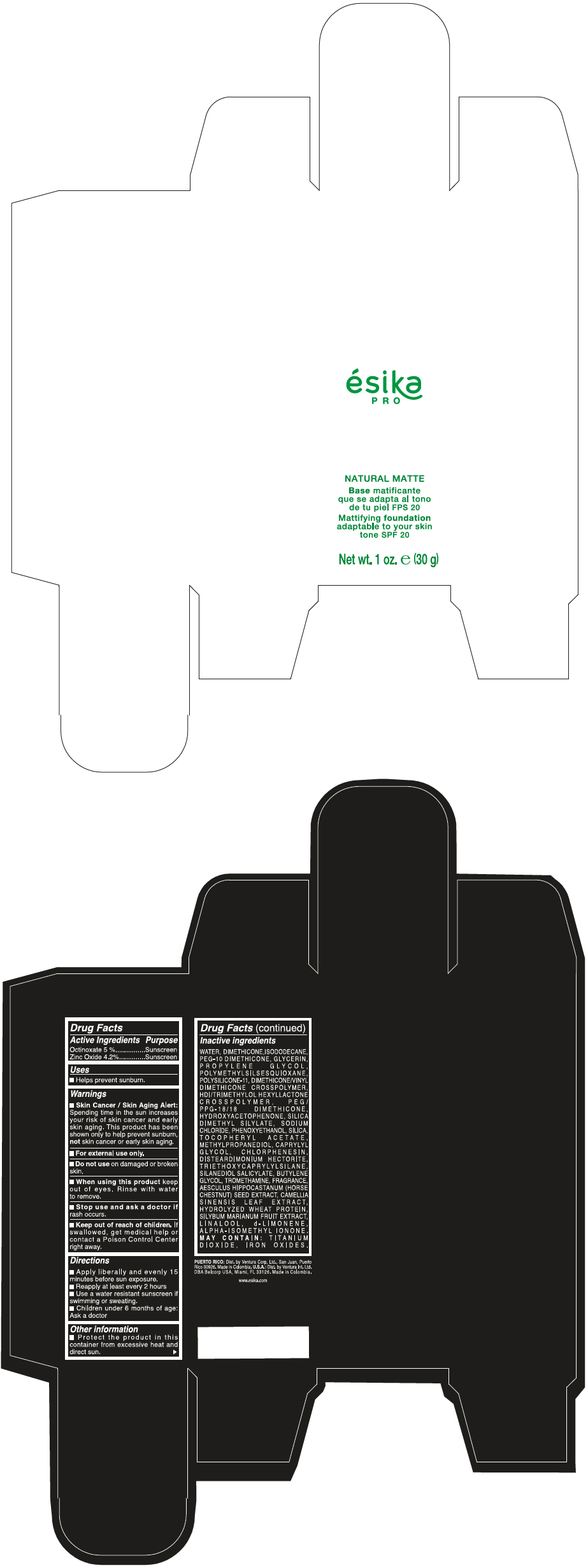
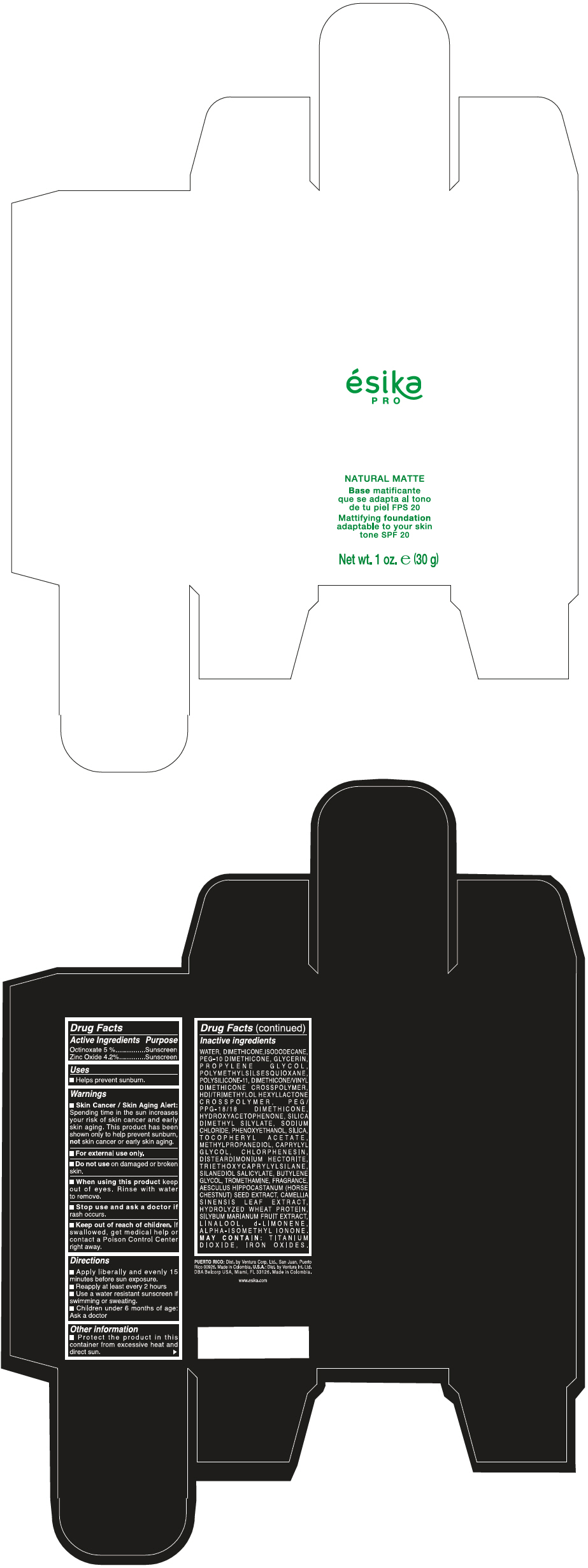
Label: ESIKA PRO NATURAL MATTIFYING FOUNDATION ADAPTABLE TO YOUR SKIN TONE SPF 20 CLARO 1/BEIGE- octinoxate and zinc oxide cream
ESIKA PRO NATURAL MATTIFYING FOUNDATION ADAPTABLE TO YOUR SKIN TONE SPF 20 CLARO 2/BEIGE- octinoxate and zinc oxide cream
ESIKA PRO NATURAL MATTIFYING FOUNDATION ADAPTABLE TO YOUR SKIN TONE SPF 20 MEDIO1/BEIGE- octinoxate and zinc oxide cream
ESIKA PRO NATURAL MATTIFYING FOUNDATION ADAPTABLE TO YOUR SKIN TONE SPF 20 MEDIO2/BEIGE- octinoxate and zinc oxide cream
ESIKA PRO NATURAL MATTIFYING FOUNDATION ADAPTABLE TO YOUR SKIN TONE SPF 20 MEDIO3/BEIGE- octinoxate and zinc oxide cream
ESIKA PRO NATURAL MATTIFYING FOUNDATION ADAPTABLE TO YOUR SKIN TONE SPF 20 MEDIO4/BEIGE- octinoxate and zinc oxide cream
ESIKA PRO NATURAL MATTIFYING FOUNDATION ADAPTABLE TO YOUR SKIN TONE SPF 20 MEDIO5/BEIGE- octinoxate and zinc oxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 43596-0119-1, 43596-0119-2, 43596-0120-1, 43596-0120-2, view more43596-0121-1, 43596-0121-2, 43596-0122-1, 43596-0122-2, 43596-0123-1, 43596-0123-2, 43596-0124-1, 43596-0124-2, 43596-0125-1, 43596-0125-2 - Packager: Ventura Corporation
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 25, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
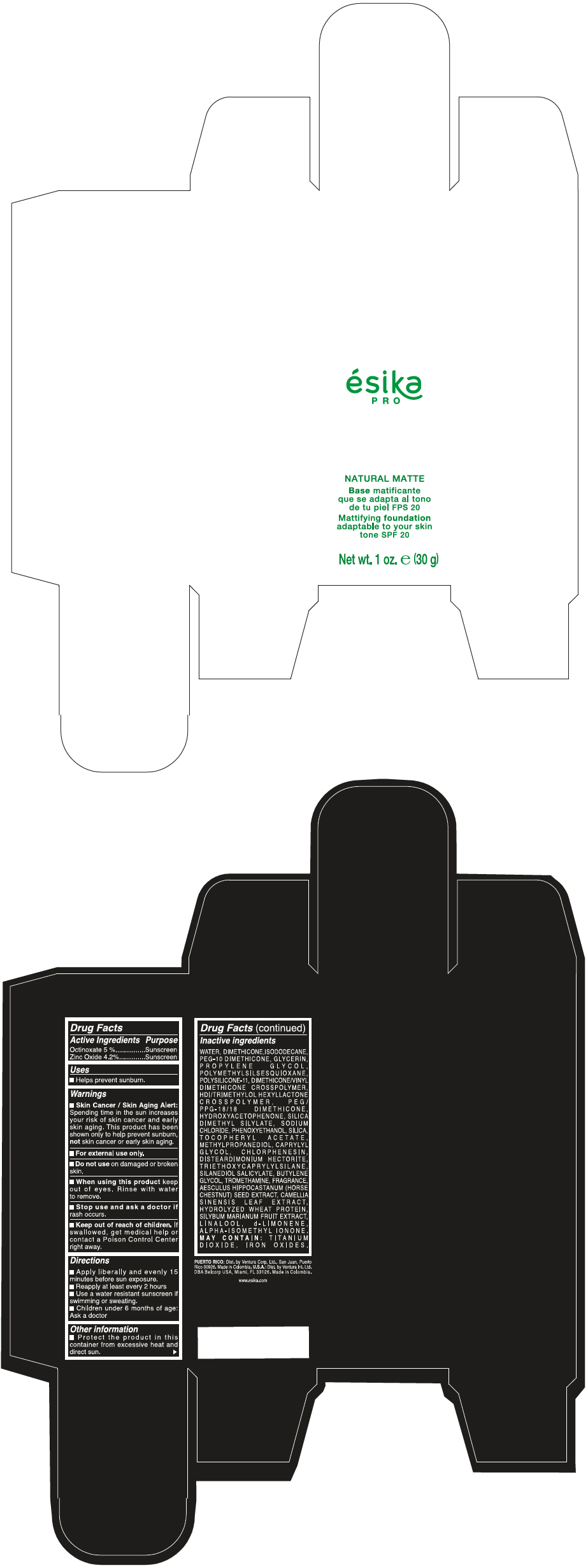
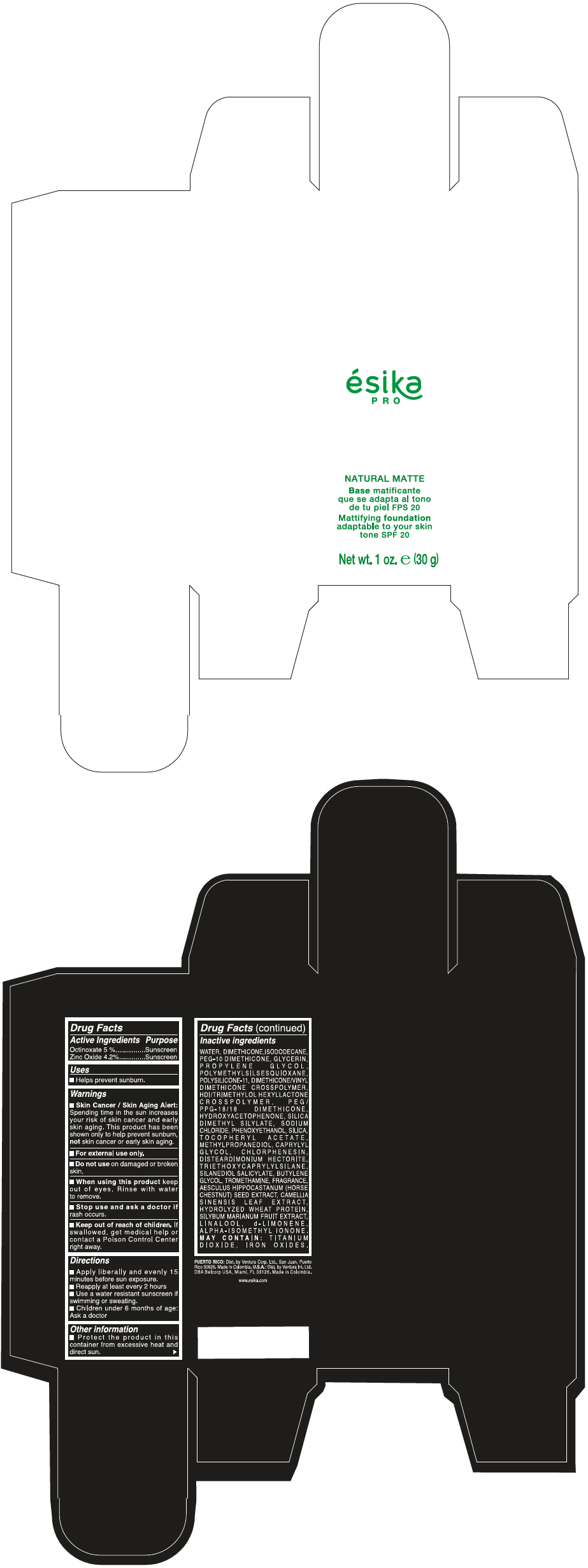
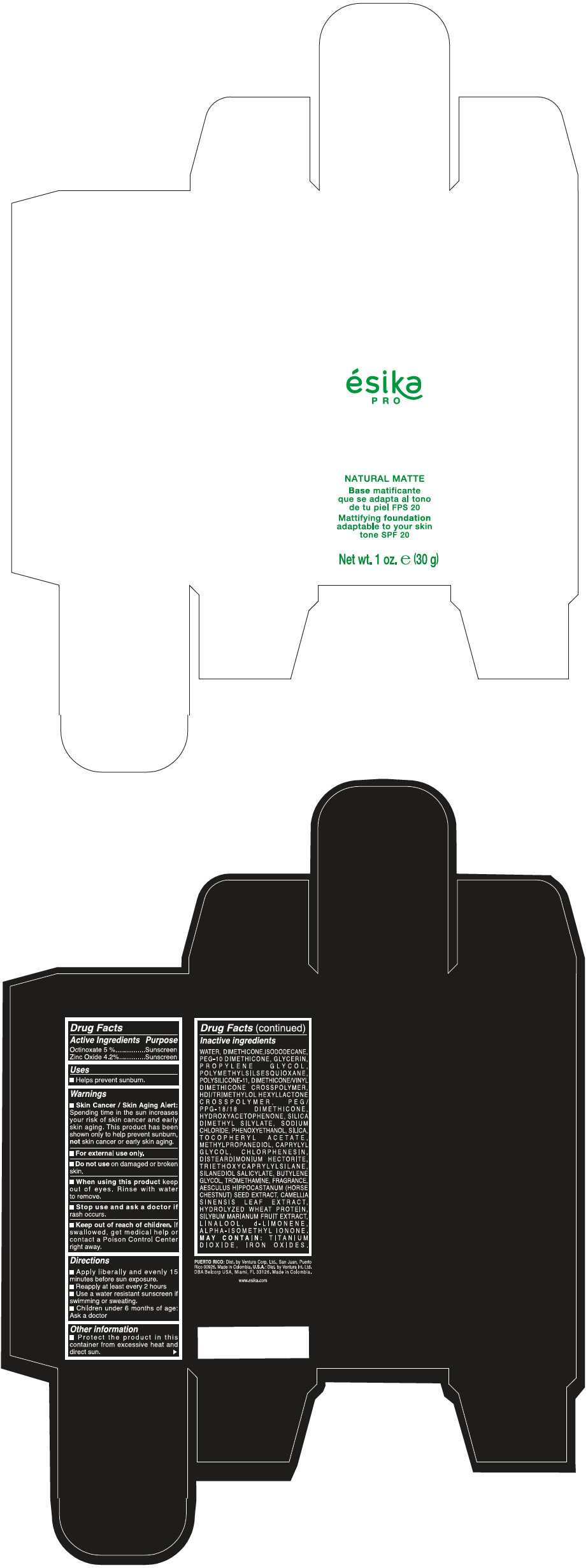
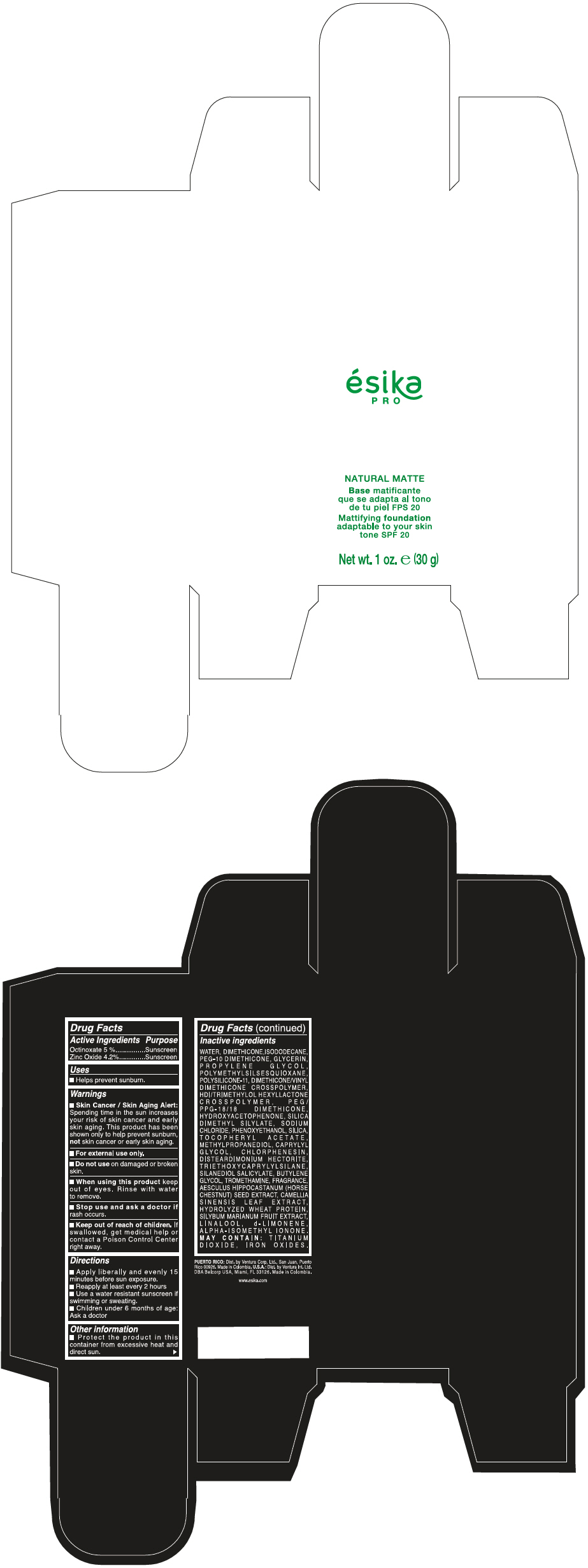
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
WATER, DIMETHICONE, ISODODECANE, PEG-10 DIMETHICONE, GLYCERIN, PROPYLENE GLYCOL, POLYMETHYLSILSESQUIOXANE, POLYSILICONE-11, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER, PEG/PPG-18/18 DIMETHICONE, HYDROXYACETOPHENONE, SILICA DIMETHYL SILYLATE, SODIUM CHLORIDE, PHENOXYETHANOL, SILICA, TOCOPHERYL ACETATE, METHYLPROPANEDIOL, CAPRYLYL GLYCOL, CHLORPHENESIN, DISTEARDIMONIUM HECTORITE, TRIETHOXYCAPRYLYLSILANE, SILANEDIOL SALICYLATE, BUTYLENE GLYCOL, TROMETHAMINE, FRAGRANCE, AESCULUS HIPPOCASTANUM (HORSE CHESTNUT) SEED EXTRACT, CAMELLIA SINENSIS LEAF EXTRACT, HYDROLYZED WHEAT PROTEIN, SILYBUM MARIANUM FRUIT EXTRACT, LINALOOL, d-LIMONENE, ALPHA-ISOMETHYL IONONE.
MAY CONTAIN : TITANIUM DIOXIDE, IRON OXIDES.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 g Bottle Box - Claro 1/Beige
- PRINCIPAL DISPLAY PANEL - 30 g Bottle Box - Claro 2/Beige
- PRINCIPAL DISPLAY PANEL - 30 g Bottle Box - Medio1/Beige
- PRINCIPAL DISPLAY PANEL - 30 g Bottle Box - Medio2/Beige
- PRINCIPAL DISPLAY PANEL - 30 g Bottle Box - Medio3/Beige
- PRINCIPAL DISPLAY PANEL - 30 g Bottle Box - Medio4/Beige
- PRINCIPAL DISPLAY PANEL - 30 g Bottle Box - Medio5/Beige
-
INGREDIENTS AND APPEARANCE
ESIKA PRO NATURAL MATTIFYING FOUNDATION ADAPTABLE TO YOUR SKIN TONE SPF 20 CLARO 1/BEIGE
octinoxate and zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43596-0125 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.05 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.042 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) ISODODECANE (UNII: A8289P68Y2) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SODIUM CHLORIDE (UNII: 451W47IQ8X) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SILANEDIOL SALICYLATE (UNII: C054DF30K0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TROMETHAMINE (UNII: 023C2WHX2V) HORSE CHESTNUT (UNII: 3C18L6RJAZ) GREEN TEA LEAF (UNII: W2ZU1RY8B0) HYDROLYZED WHEAT PROTEIN (ENZYMATIC; 3000 MW) (UNII: J2S07SB0YL) MILK THISTLE (UNII: U946SH95EE) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43596-0125-2 1 in 1 BOX 10/19/2018 1 NDC:43596-0125-1 30 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 10/19/2018 ESIKA PRO NATURAL MATTIFYING FOUNDATION ADAPTABLE TO YOUR SKIN TONE SPF 20 CLARO 2/BEIGE
octinoxate and zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43596-0119 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.05 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.042 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) ISODODECANE (UNII: A8289P68Y2) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SODIUM CHLORIDE (UNII: 451W47IQ8X) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SILANEDIOL SALICYLATE (UNII: C054DF30K0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TROMETHAMINE (UNII: 023C2WHX2V) HORSE CHESTNUT (UNII: 3C18L6RJAZ) GREEN TEA LEAF (UNII: W2ZU1RY8B0) HYDROLYZED WHEAT PROTEIN (ENZYMATIC; 3000 MW) (UNII: J2S07SB0YL) MILK THISTLE (UNII: U946SH95EE) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43596-0119-2 1 in 1 BOX 10/19/2018 1 NDC:43596-0119-1 30 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 10/19/2018 ESIKA PRO NATURAL MATTIFYING FOUNDATION ADAPTABLE TO YOUR SKIN TONE SPF 20 MEDIO1/BEIGE
octinoxate and zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43596-0120 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.05 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.042 g in 1 g Inactive Ingredients Ingredient Name Strength GREEN TEA LEAF (UNII: W2ZU1RY8B0) HYDROLYZED WHEAT PROTEIN (ENZYMATIC; 3000 MW) (UNII: J2S07SB0YL) MILK THISTLE (UNII: U946SH95EE) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) ISODODECANE (UNII: A8289P68Y2) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SODIUM CHLORIDE (UNII: 451W47IQ8X) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SILANEDIOL SALICYLATE (UNII: C054DF30K0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TROMETHAMINE (UNII: 023C2WHX2V) HORSE CHESTNUT (UNII: 3C18L6RJAZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43596-0120-2 1 in 1 BOX 10/19/2018 1 NDC:43596-0120-1 30 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 10/19/2018 ESIKA PRO NATURAL MATTIFYING FOUNDATION ADAPTABLE TO YOUR SKIN TONE SPF 20 MEDIO2/BEIGE
octinoxate and zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43596-0121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.05 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.042 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) ISODODECANE (UNII: A8289P68Y2) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SODIUM CHLORIDE (UNII: 451W47IQ8X) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SILANEDIOL SALICYLATE (UNII: C054DF30K0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TROMETHAMINE (UNII: 023C2WHX2V) HORSE CHESTNUT (UNII: 3C18L6RJAZ) GREEN TEA LEAF (UNII: W2ZU1RY8B0) HYDROLYZED WHEAT PROTEIN (ENZYMATIC; 3000 MW) (UNII: J2S07SB0YL) MILK THISTLE (UNII: U946SH95EE) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43596-0121-2 1 in 1 BOX 10/19/2018 1 NDC:43596-0121-1 30 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 10/19/2018 ESIKA PRO NATURAL MATTIFYING FOUNDATION ADAPTABLE TO YOUR SKIN TONE SPF 20 MEDIO3/BEIGE
octinoxate and zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43596-0122 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.05 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.042 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) ISODODECANE (UNII: A8289P68Y2) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SODIUM CHLORIDE (UNII: 451W47IQ8X) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SILANEDIOL SALICYLATE (UNII: C054DF30K0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TROMETHAMINE (UNII: 023C2WHX2V) HORSE CHESTNUT (UNII: 3C18L6RJAZ) GREEN TEA LEAF (UNII: W2ZU1RY8B0) HYDROLYZED WHEAT PROTEIN (ENZYMATIC; 3000 MW) (UNII: J2S07SB0YL) MILK THISTLE (UNII: U946SH95EE) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43596-0122-2 1 in 1 BOX 10/19/2018 1 NDC:43596-0122-1 30 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 10/19/2018 ESIKA PRO NATURAL MATTIFYING FOUNDATION ADAPTABLE TO YOUR SKIN TONE SPF 20 MEDIO4/BEIGE
octinoxate and zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43596-0123 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.05 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.042 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) ISODODECANE (UNII: A8289P68Y2) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SODIUM CHLORIDE (UNII: 451W47IQ8X) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SILANEDIOL SALICYLATE (UNII: C054DF30K0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TROMETHAMINE (UNII: 023C2WHX2V) HORSE CHESTNUT (UNII: 3C18L6RJAZ) GREEN TEA LEAF (UNII: W2ZU1RY8B0) HYDROLYZED WHEAT PROTEIN (ENZYMATIC; 3000 MW) (UNII: J2S07SB0YL) MILK THISTLE (UNII: U946SH95EE) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43596-0123-2 1 in 1 BOX 10/19/2018 1 NDC:43596-0123-1 30 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 10/19/2018 ESIKA PRO NATURAL MATTIFYING FOUNDATION ADAPTABLE TO YOUR SKIN TONE SPF 20 MEDIO5/BEIGE
octinoxate and zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43596-0124 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.05 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.042 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) ISODODECANE (UNII: A8289P68Y2) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SODIUM CHLORIDE (UNII: 451W47IQ8X) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SILANEDIOL SALICYLATE (UNII: C054DF30K0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TROMETHAMINE (UNII: 023C2WHX2V) HORSE CHESTNUT (UNII: 3C18L6RJAZ) GREEN TEA LEAF (UNII: W2ZU1RY8B0) HYDROLYZED WHEAT PROTEIN (ENZYMATIC; 3000 MW) (UNII: J2S07SB0YL) MILK THISTLE (UNII: U946SH95EE) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43596-0124-2 1 in 1 BOX 10/19/2018 1 NDC:43596-0124-1 30 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 10/19/2018 Labeler - Ventura Corporation (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 manufacture(43596-0125, 43596-0119, 43596-0120, 43596-0121, 43596-0122, 43596-0123, 43596-0124)