Label: GOLD DYNAMICS SKIN BOOSTING MOISTURIZER BROAD SPECTRUM SPF 15 SUNSCREEN- avobenzone, oxybenzone, octisalate, octinoxate cream
- NDC Code(s): 68828-099-01, 68828-099-02
- Packager: Jafra Cosmetics International Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
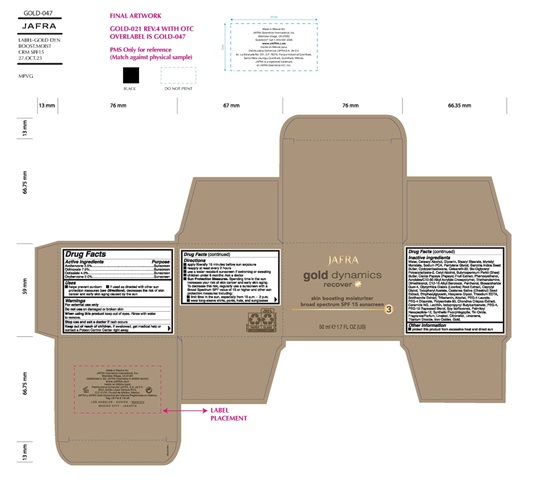
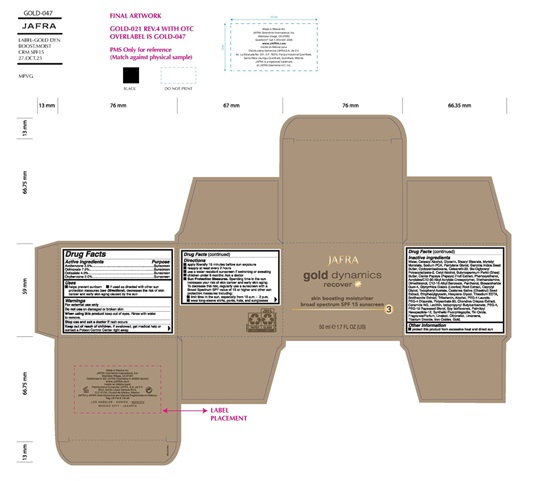
- Active ingredient
- Purpose
- Uses
- Warning
-
Direction
• Apply liberally 15 minutes before sun exposure
• Use a water resistant sunscreen if swimming or sweating
• Reapply at least every 2 hours
• Children under 6 months of age: Ask a doctor
• Sun Protection Measures.Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit your time in the sun, especially from 10 a.m. – 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses - KEEP OUT OF REACH OF CHILDREN
-
Inactive ingredients
Water, Cetearyl Alcohol, Glycerin, Stearyl Stearate, Myristyl Myristate, Sodium PCA, Pentylene Glycol, Garcinia Indica Seed Butter, Ceteareth-20, Bis-Diglyceryl Polyacyladipate-2, Cyclomethicone, Cetyl Alcohol, Butyrospermum Parkii (Shea) Butter, Carica Papaya (Papaya) Fruit Extract, Castanea Sativa (Chestnut) Seed Extract, Glycyrrhiza Glabra (Licorice) Root Extract, Chondrus Crispus Extract, Soy Isoflavones, Dimethiconol, Gold, Ceramide NG, Phenoxyethanol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Triethanolamine, C12-15 Alkyl Benzoate, Panthenol, Biosaccharide Gum-4, Caprylyl Glycol, Tocopheryl Acetate, Ethylhexylglycerin, Hexylene Glycol, Trisodium EDTA, Smithsonite Extract, Tribehenin, Alcohol, PEG-4 Laurate, PEG-4 Dilaurate, Polysorbate 80, Lecithin, Iodopropynyl Butylcarbamate, PEG-4, PEG-10 Phytosterol, Palmitoyl Hexapeptide-12, Fragrance, Synthetic Fluorphlogopite, Titanium Dioxide, Iron Oxides, Tin Oxide
- Other information
- Product label
-
INGREDIENTS AND APPEARANCE
GOLD DYNAMICS SKIN BOOSTING MOISTURIZER BROAD SPECTRUM SPF 15 SUNSCREEN
avobenzone, oxybenzone, octisalate, octinoxate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68828-099 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.5 g in 100 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 2 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4 g in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERIN (UNII: PDC6A3C0OX) STEARYL STEARATE (UNII: 5WX2EGD0DK) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) PENTYLENE GLYCOL (UNII: 50C1307PZG) GARCINIA INDICA SEED BUTTER (UNII: US2H3D7800) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) CYCLOMETHICONE (UNII: NMQ347994Z) CETYL ALCOHOL (UNII: 936JST6JCN) SHEA BUTTER (UNII: K49155WL9Y) PAPAYA (UNII: KU94FIY6JB) SPANISH CHESTNUT (UNII: 2MT5XMR2YW) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) CHONDRUS CRISPUS CARRAGEENAN (UNII: UE856F2T78) SOY ISOFLAVONES (UNII: 71B37NR06D) DIMETHICONE (UNII: 92RU3N3Y1O) GOLD (UNII: 79Y1949PYO) CERAMIDE NG (UNII: C04977SRJ5) PHENOXYETHANOL (UNII: HIE492ZZ3T) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) TROLAMINE (UNII: 9O3K93S3TK) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PANTHENOL (UNII: WV9CM0O67Z) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) CAPRYLYL GLYCOL (UNII: 00YIU5438U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HEXYLENE GLYCOL (UNII: KEH0A3F75J) EDETATE TRISODIUM (UNII: 420IP921MB) TRIBEHENIN (UNII: 8OC9U7TQZ0) ALCOHOL (UNII: 3K9958V90M) PEG-4 LAURATE (UNII: AYF4VM3N1Z) PEG-4 DILAURATE (UNII: KCR71CW036) POLYSORBATE 80 (UNII: 6OZP39ZG8H) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) POLYETHYLENE GLYCOL 200 (UNII: R95B8J264J) PALMITOYL HEXAPEPTIDE-12 (UNII: HO4ZT5S86C) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROUS OXIDE (UNII: G7036X8B5H) STANNOUS OXIDE (UNII: JB2MV9I3LS) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-099-01 1 in 1 CARTON 04/13/2022 1 200 mL in 1 JAR; Type 0: Not a Combination Product 2 NDC:68828-099-02 1 in 1 CARTON 04/13/2022 2 50 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/13/2022 Labeler - Jafra Cosmetics International Inc (041676479) Registrant - Jafra Cosmetics International Inc (041676479) Establishment Name Address ID/FEI Business Operations Distribuidora Comercial Jafra, S.A. de C.V. 951612777 manufacture(68828-099)