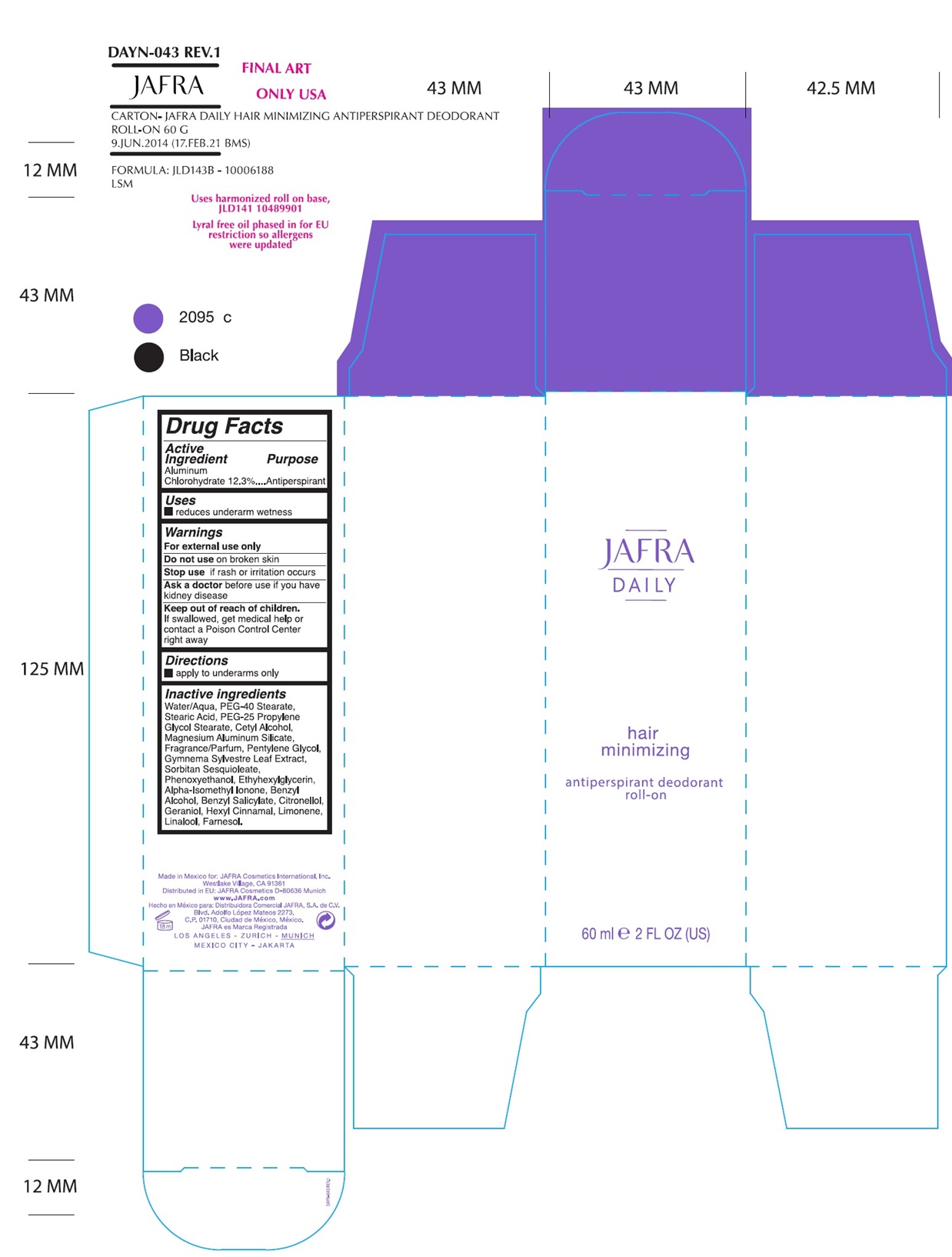
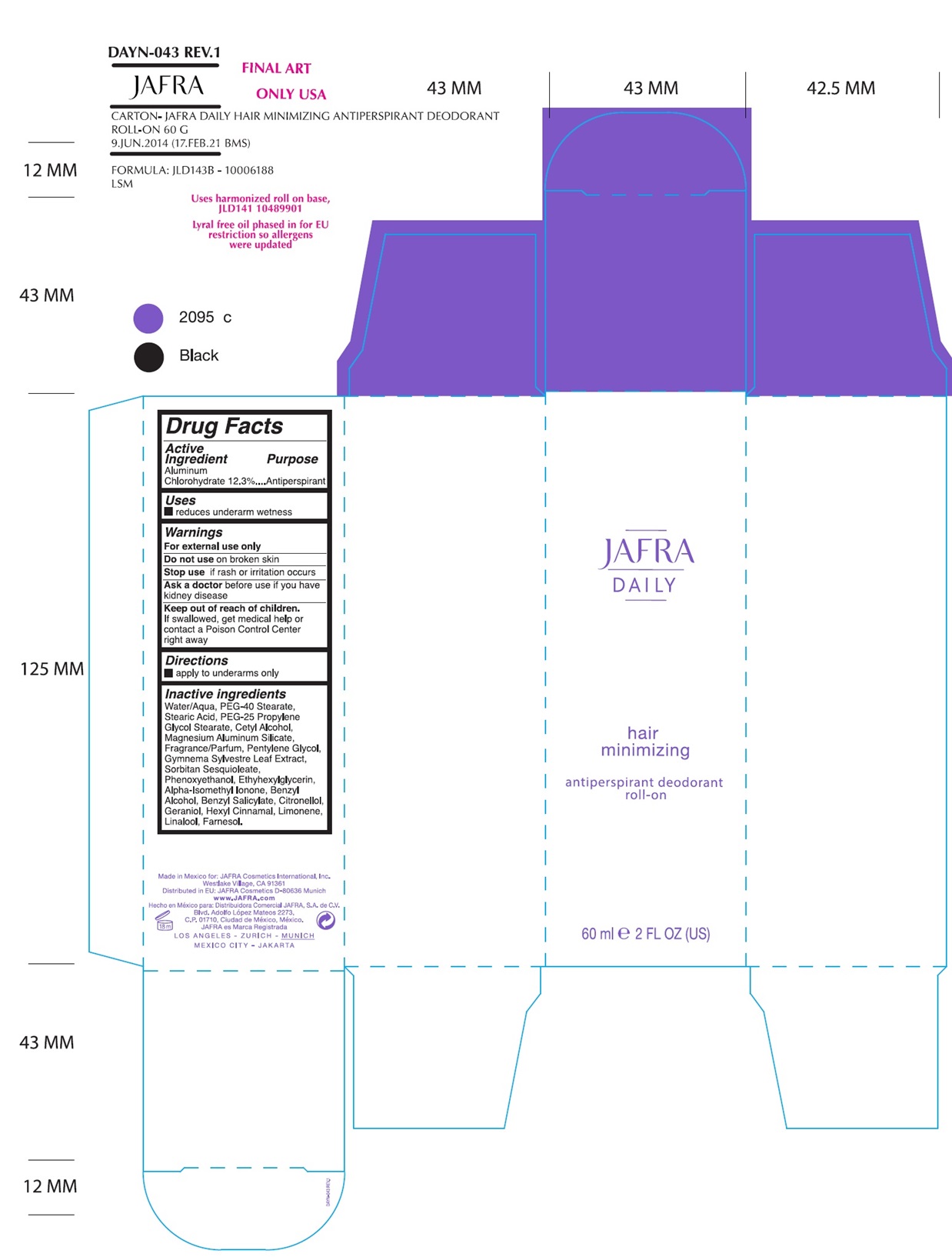
Label: JAFRA DAILY HAIR MINIMIZING ANTIPERSPIRANT DEODORANT ROLL-ON- aluminum chlorohydrate liquid
- NDC Code(s): 68828-703-01, 68828-703-02
- Packager: Jafra Cosmetics International
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Directions
-
Inactive Ingredients
Water/Aqua, PEG-40 Stearate, Stearic Acid, PEG-25 Propylene Glycol Stearate, Cetyl Alcohol, Magnesium Aluminum Silicate, Fragrance/Parfum, Pentylene Glycol, Gymnema Sylvestre Leaf Extract, Sorbitan Sesquioleate, Phenoxyethanol, Ethylhexylglycerin, Alpha-Isomethyl Ionone, Benzyl Alcohol, Benzyl Salicylate, Citronellol, Geraniol, Hexyl Cinnamal, Limonene, Linalool, Farnesol
- Product label
-
INGREDIENTS AND APPEARANCE
JAFRA DAILY HAIR MINIMIZING ANTIPERSPIRANT DEODORANT ROLL-ON
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68828-703 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 12.3 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PEG-40 STEARATE (UNII: ECU18C66Q7) PEG-25 PROPYLENE GLYCOL STEARATE (UNII: X21KPH4633) STEARIC ACID (UNII: 4ELV7Z65AP) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) CETYL ALCOHOL (UNII: 936JST6JCN) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) PENTYLENE GLYCOL (UNII: 50C1307PZG) GYMNEMA SYLVESTRE LEAF (UNII: 2ZK6ZS8392) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) BENZYL ALCOHOL (UNII: LKG8494WBH) BENZYL SALICYLATE (UNII: WAO5MNK9TU) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) GERANIOL (UNII: L837108USY) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) FARNESOL (UNII: EB41QIU6JL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-703-02 1 in 1 CARTON 12/31/2021 1 NDC:68828-703-01 60 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 12/31/2021 Labeler - Jafra Cosmetics International (041676479) Registrant - Jafra Cosmetics International (041676479) Establishment Name Address ID/FEI Business Operations Distribuidora Comercial Jafra, S.A. de C.V. 951612777 manufacture(68828-703)