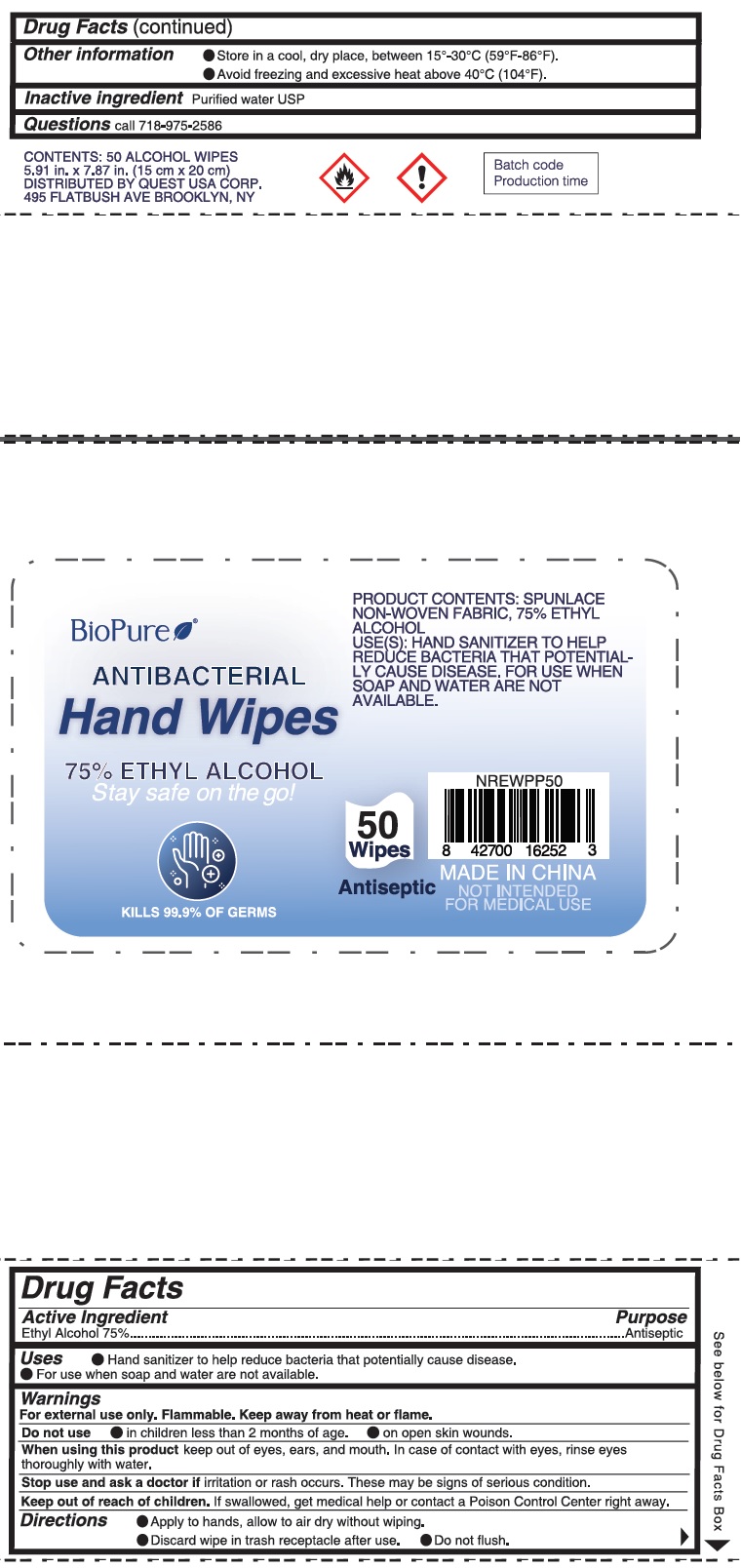
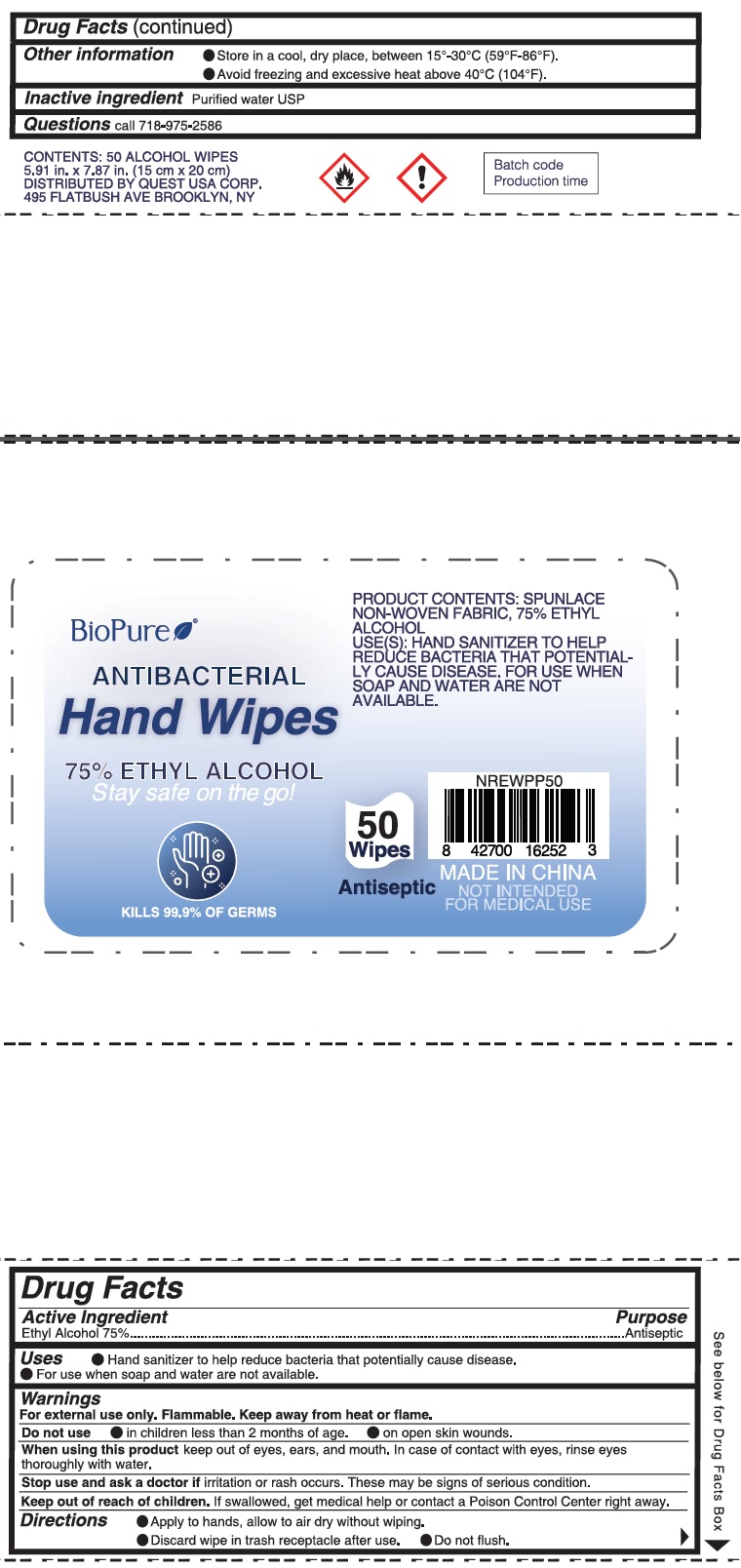
Label: BIOPURE ANTIBACTERIAL HAND WIPE- benzalkonium chloride cloth
- NDC Code(s): 78691-004-00, 78691-004-01, 78691-004-02, 78691-004-03
- Packager: QUEST USA CORP.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Question?
- Package Labeling:1600ct
- Package Labeling:800ct
- Package Labeling:50ct
- Package Labeling:100ct
-
INGREDIENTS AND APPEARANCE
BIOPURE ANTIBACTERIAL HAND WIPE
benzalkonium chloride clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:78691-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.2 mg in 1 mL Inactive Ingredients Ingredient Name Strength PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78691-004-00 1600 in 1 BAG 09/15/2020 1 3.44 mL in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:78691-004-01 800 in 1 BAG 09/15/2020 10/09/2022 2 3.25 mL in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:78691-004-02 50 in 1 POUCH 09/15/2020 09/14/2022 3 5.7 mL in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 4 NDC:78691-004-03 100 in 1 CANISTER 09/15/2020 09/22/2022 4 5.37 mL in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 09/15/2020 Labeler - QUEST USA CORP. (079869689)