Label: NITROGEN gas
- NDC Code(s): 74709-002-01
- Packager: Air Liquide Canada Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved medical gas
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Air Liquide Canada Nitrogen
Air Liquide
NITROGEN, AZOTE
COMPRESSED COMPRIME
1066
2
NITROGEN / AZOTE
WARNING: CONTYAINS GAS UNDER PRESSURE; MAY EXPLODE IF HEATED. MAY DISPLACE OXYGEN AND CAUSE RAPID SUFFOCATION. Do not handle until all safety precautions have been read and understood. Use and store only outdoors or in a well-ventilated place. Use a back flow preventive device in the piping. Use only with equipment rated for cylinder pressure. Close valve after each use and when empty. Protect from sunlight when ambient temperature exceeds 52°C (125°F). Read and follow the Safety Data Sheet (SDS) before use. FIRST AID; INHALED: Remove person to fresh air and keep comfortable for breathing. Get medical advice/attention.
ATTENTION: CONTIENT UNGAZ SOUS PRESSION; PEUT EXPLOSER SOUS L’EFFET DE LACHALEUR, PEUT SE SUBSTITUER A L’OXGENE ET CAUSER RAPIDEMENT LA SUFFOCATION. Ne pas manipuler avant d’avoir lu et compris toutes les precautins de securite. N’utiliser et e’entreposer qu’a l’exterieur ou dans un endroit bien ventile. Poser un dispositive anti retour dans le circuit de conduits. Utilser uniquement avec l’equipment estime pour la pression de cylinder. Fermer le robinet apres chaque utilization et lorsque le contenant est vide. Proteger contre les rayons du solell lorsque la temperature ambiante depasse 52°C (125°F). Lire et suivre la tiche signatetique (FS) avant d’utiliser. PREMIERS SOINS; EN CAS D’INHALATION; Transporter la personnne a l’exterieur et la maintenir dans une position ou elle peut confortablement respire. Demander un avis medical /Consultr un medecin.
DO NOT REMOVE THIS LABEL NE PAS ENLEVER CETTE ÉTIQUETTE.
™AIR LIQUIDE CANADA INC.
1250 René-Levesque West, Suite 1700, Montreal, OC H3B 5E6 Canada
1-514-878-1667

-
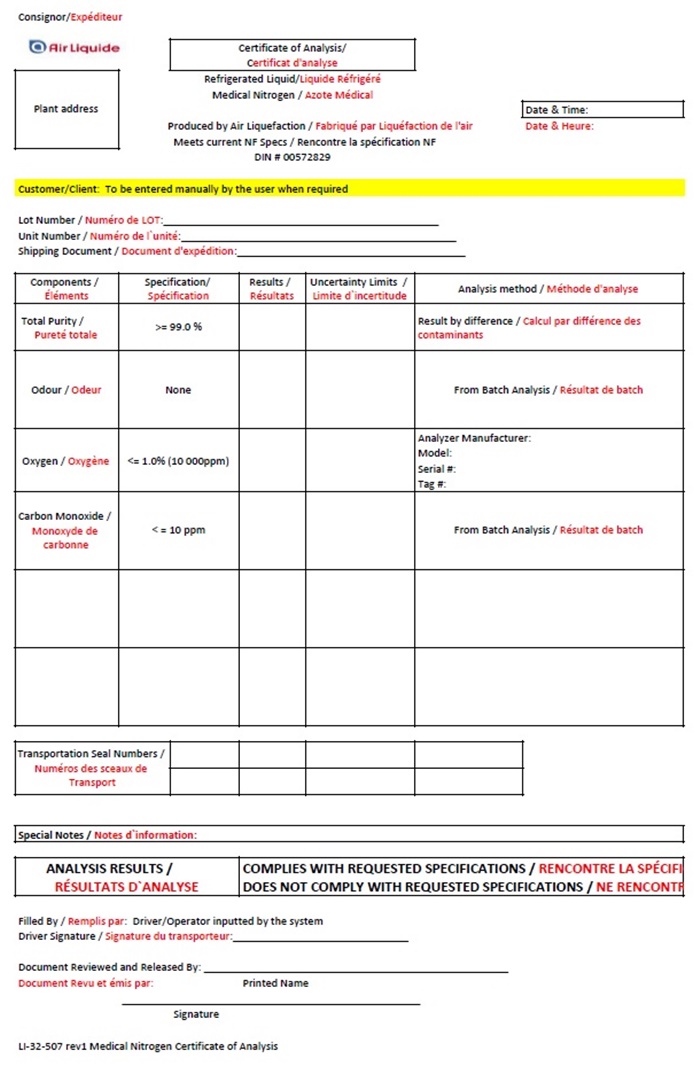
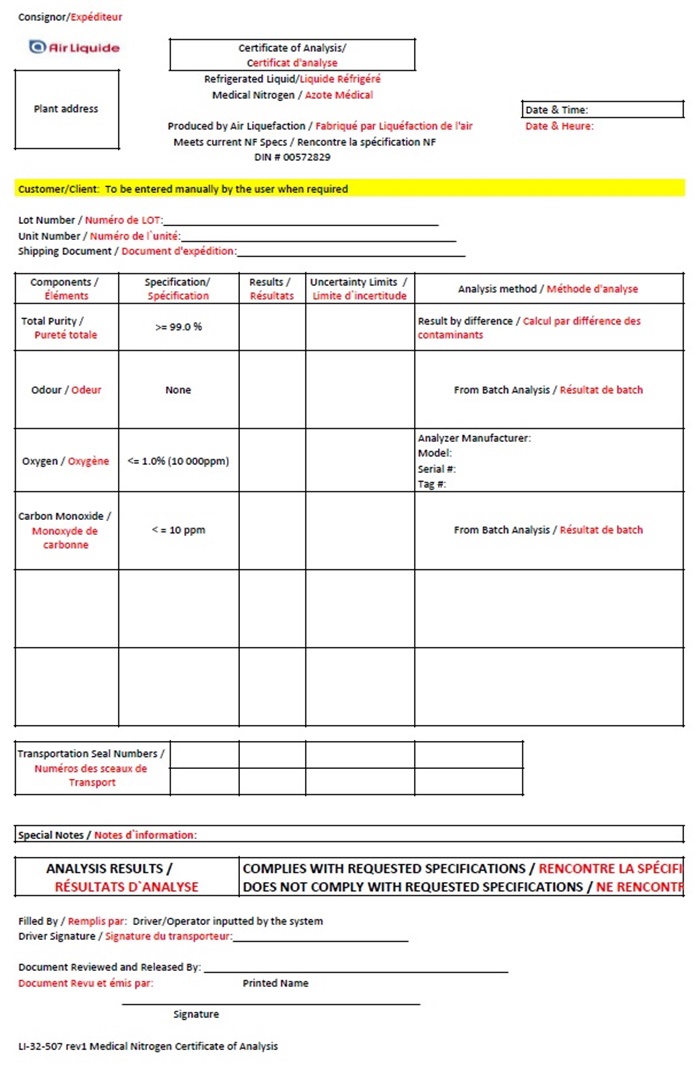
Air Liquide Canada Nitrogen COA
Consignor/ Expèditeur
Air Liquide Certificate of Analysis
Certificat d’analyse
Plant Address Refrigerated Liquid / Liquide Réfrigéré
Medical Nitrogen / Azote Médical
Date & Time
Date & Heure:
Produced by Air Liquefaction / Fabriqué par Liquéfaction de l’air
Meets current NF Specs / Rencontre la spécifcation NF
DIN # 00572829
Customer/Client: To be entered manually by the user when required
Lot Number / Numéro de LOT: __________________
Unit Number / Numéro de l’unité: _______________
Shipping Document / Document d’expédition: ________________
Componets /
Éléments
Specification /
Specification
Results /
Résultats
Uncertainty Limits /
Limite d’incertitude
Analysis method / Méthode d’analyse
Total Purity / Pureté totale
>=99.0%
Result by difference / Calcul par difference des contaminants
Odour / Odeur
None
From Batch Analysis / Résultat de batch
Oxygen / Oxygéne
<=1.0% (10000ppm)
Analyzer Manufacturer:
Model:
Serial #:
Tag #:
Carbon Monoxide / Monoxyde de carbonne
<=10 ppm
From Batch Analysis / Résultat de batch
Transportation Seal Numbers / Numéros des sceaux de Transport
Special Notes / Notes d’information:
ANALYSIS RESULTS /
RÉSULTATS D’ANALYSE
COMPLIES WITH REQUESTED SPECIFICATIONS / RENCONTRE LA SPÉCIFICATION
DOES NOT COMPLY WITH REQUESTED SPECIFICATIONS / NE RECONTR
Filled By / Remplis par: Driver / Operator inputted by the system
Driver Signature / Signature du transporteur: ______________________________
Document Reviewed and Released By: ___________________________________
Printed Name
Document Revu et émis par:
______________________________________________________
Signature
LI-32-507 rev 1 Medical Nitrogen Certificate of Analysis
-
INGREDIENTS AND APPEARANCE
NITROGEN
nitrogen gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:74709-002 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITROGEN (UNII: N762921K75) (NITROGEN - UNII:N762921K75) NITROGEN 992 mL in 1 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74709-002-01 25738 L in 1 TANK; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved medical gas 05/01/2020 Labeler - Air Liquide Canada Inc (251676490) Establishment Name Address ID/FEI Business Operations Air Liquide Canada Inc 251676490 manufacture(74709-002)