Label: ARUBA ALOE ISLAND REMEDY DAILY ULTRA ALOE- octinoxate, oxybenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 53675-100-01 - Packager: ARUBA ALOE BALM NV
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 7, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
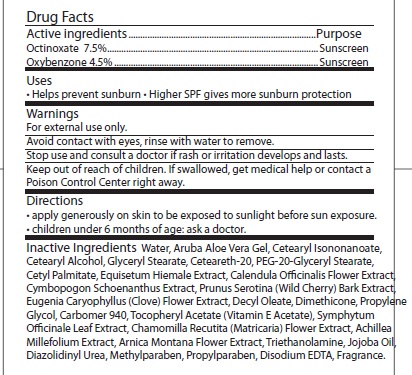
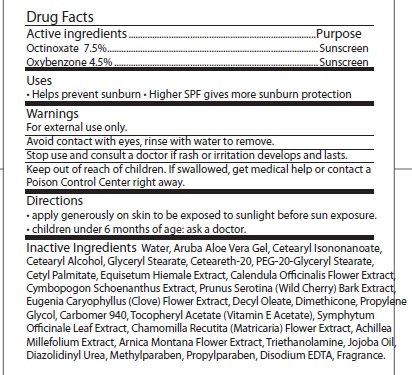
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive Ingredients: WATER, ARUBA ALOE VERA GEL, CETEARYL ISONONANOATE, CETEARYL ALCOHOL, GLYCERYL STEARATE, CETEARETH-20, PEG-20-GLYCERYL STEARATE, CETYL PALMITATE, EQUISETUM HIEMALE EXTRACT, CALENDULA OFFICINALIS FLOWER EXTRACT, CYMBOPOGON SCHOENANTHUS EXTRACT, PRUNUS SEROTINA (WILD CHERRY) BARK EXTRACT, EUGENIA CARYOPHYLLUS (CLOVE) FLOWER EXTRACT, DECYL OLEATE, DIMETHICONE, PROPYLENE GLYCOL, CARBOMER 940, TOCOPHERYL ACETATE (VITAMIN E ACETATE), SYMPHYTUM OFFICINALE LEAF EXTRACT, CHAMOMILLA RECUTITA (MATRICARIA) FLOWER EXTRACT, ACHILLEA MILLEFOLIUM EXTRACT, ARNICA MONTANA FLOWER EXTRACT, TRIETHANOLAMINE, JOJOBA OIL, DIAZOLIDINYL UREA, METHYLPARABEN, PROPYLPARABEN, DISODIUM EDTA, FRAGRANCE.
-
PRINCIPAL DISPLAY PANEL
ARUBA ALOE
ISLAND REMEDY
Island Remedy products are not tested on animals. MADE IN ARUBA BY ARUBA ALOE BALM INC PO BOX 360 ARUBA DUTCH CARIBBEAN
DISTRIBUTED IN THE USA BY ARUBA ALOE OF NORTH AMERICA L.L.C INDIANAPOLIS IN 46250
ARUBA ALOE
THE WORLDS FINEST ALOE
ISLAND REMEDY
SINCE 1890
DAILY ULTRA ALOE CREME SPF 15
A NATURAL ADVANTAGE
Deeply rooted in the shadow of the Watapana tree, an earthy island remedy thrives. Since 1890, it as grown into an entire line of skin care products known around the world for its moisturizing benefits. Experience the Aruba Aloe difference.
ARUBA ALOE ISLAND REMEDY
DAILY ULTRA ALOE CREME
SPF 15
4OZ 118 ML
DAILY ULTRA ALOE CREME
SPF 15
Essential for all-day protection, this powerful moisturizing creme is formulated with 10% Pure Aruba Aloe Vera, SPF 15 sunscreens. Comfrey and Arnica extracts to help neutralize the negative effects of the sun's rays while stimulating your skin's elasticity and cell-reviving capabilities. Helps prevent sunburn. Higher SPF gives more sunburn protection. for skin that sunburns easily.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ARUBA ALOE ISLAND REMEDY DAILY ULTRA ALOE
octinoxate, oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53675-100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) CETEARYL ISONONANOATE (UNII: P5O01U99NI) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CETYL PALMITATE (UNII: 5ZA2S6B08X) EQUISETUM HYEMALE (UNII: 59677RXH25) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) PRUNUS SEROTINA BARK (UNII: 5D48E975HA) CLOVE (UNII: K48IKT5321) DECYL OLEATE (UNII: ZGR06DO97T) DIMETHICONE (UNII: 92RU3N3Y1O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOMER HOMOPOLYMER TYPE C (UNII: 4Q93RCW27E) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) TROLAMINE (UNII: 9O3K93S3TK) JOJOBA OIL (UNII: 724GKU717M) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) EDETATE DISODIUM (UNII: 7FLD91C86K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53675-100-01 1 in 1 BOX 11/01/2010 1 118 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2010 Labeler - ARUBA ALOE BALM NV (855442273)