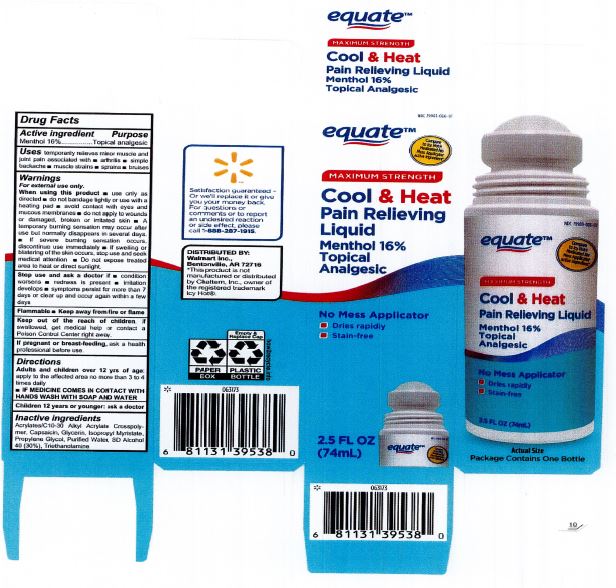
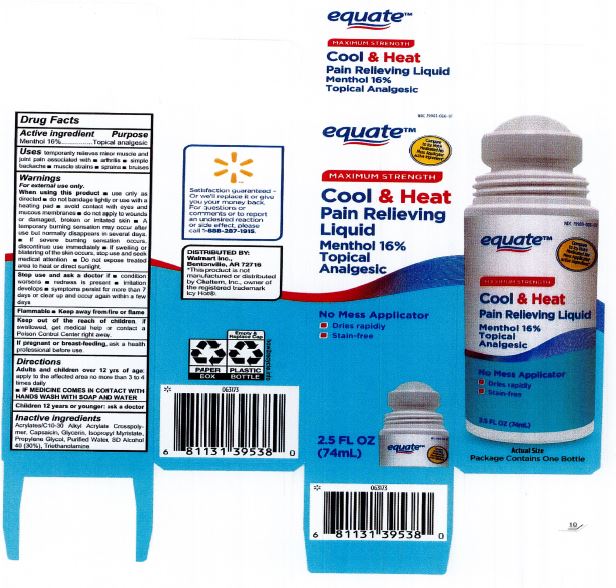
Label: EQUATE COOL AND HEAT PAIN RELIEVING LIQUID- cool and heat pain relief liquid
- NDC Code(s): 79903-006-97
- Packager: Walmart, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
-
WARNINGS
When using this product: use only as directed. Do not bandage tightly or use with a heating pad. Avoid contact with eyes and mucous membranes. Do not apply to wounds or damaged, broken or irritated skin. A temporary burning sensation may occur after use but normally disappears in several days. If severe burning sensation occurs, discontinue use immediately. If swelling or blistering of the skin occurs, stop use and seek medical attention. Do not expose treated area to heat or direct sunlight.
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- ACTIVE INGREDIENT
- PREGNANCY OR BREAST FEEDING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EQUATE COOL AND HEAT PAIN RELIEVING LIQUID
cool and heat pain relief liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 16 mg in 100 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) (C10-C30)ALKYL METHACRYLATE ESTER (UNII: XH2FQZ38D8) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TROLAMINE (UNII: 9O3K93S3TK) ALCOHOL (UNII: 3K9958V90M) CAPSAICIN (UNII: S07O44R1ZM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-006-97 74 mL in 1 CONTAINER; Type 0: Not a Combination Product 08/17/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 08/17/2020 Labeler - Walmart, Inc. (051957769) Registrant - Pharma Nobis, LLC (118564114) Establishment Name Address ID/FEI Business Operations Pharma Nobis, LLC 118564114 label(79903-006) , pack(79903-006) , analysis(79903-006) , manufacture(79903-006)