Label: BENDEKA- bendamustine hydrochloride injection, solution
- NDC Code(s): 63459-348-04
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BENDEKA safely and effectively. See full prescribing information for BENDEKA.
BENDEKA® (bendamustine hydrochloride injection), for intravenous use
Initial U.S. Approval: 2008
INDICATIONS AND USAGE
BENDEKA injection is an alkylating drug indicated for treatment of patients with:
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Injection: 100 mg/4 mL (25 mg/mL) in a multiple-dose vial. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Myelosuppression: Delay or reduce dose, and restart treatment based on ANC and platelet count recovery. (2.1, 5.1)
- Infections: Monitor for fever and other signs of infection or reactivation of infections and treat promptly. (5.2)
- Progressive multifocal leukoencephalopathy (PML): Monitor for new or worsening neurological, cognitive or behavioral signs or symptoms suggestive of PML. (5.3)
- Anaphylaxis and Infusion Reactions: Severe anaphylactic reactions have occurred. Monitor clinically and discontinue drug for severe reactions. Pre-medicate in subsequent cycles for milder reactions. (5.4)
- Tumor Lysis Syndrome: May lead to acute renal failure and death; anticipate and use supportive measures in patients at high risk. (5.5)
- Skin Reactions: Discontinue for severe skin reactions. Cases of SJS, DRESS and TEN, some fatal, have been reported. (5.6).
- Hepatotoxicity: Monitor liver chemistry tests prior to and during treatment. (5.7)
- Other Malignancies: Pre-malignant and malignant diseases have been reported. (5.8)
- Extravasation Injury: Take precautions to avoid extravasation, including monitoring intravenous infusion site during and after administration. (5.9)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential and males with female partners of reproductive potential of the potential risk to a fetus and to use an effective method of contraception. (5.10, 8.1, 8.3)
ADVERSE REACTIONS
- Adverse reactions (frequency >5%) during infusion and within 24 hours post-infusion are nausea and fatigue. (6.1)
- Most common adverse reactions (≥15%) for CLL are anemia, thrombocytopenia, neutropenia, lymphopenia, leukopenia, hyperbilirubinemia, pyrexia, nausea, vomiting. (6.1, 6.2)
- Most common adverse reactions (≥15%) for NHL are lymphopenia, leukopenia, anemia, neutropenia, thrombocytopenia, nausea, fatigue, vomiting, diarrhea, pyrexia, constipation, anorexia, cough, headache, weight decreased, dyspnea, rash, and stomatitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals at 1-888-483-8279 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Consider alternative therapies that are not CYP1A2 inducers or inhibitors during treatment with BENDEKA. (7.1)
USE IN SPECIFIC POPULATIONS
- Lactation: Advise not to breastfeed. (8.2)
- Infertility: May impair fertility. (8.3)
- Renal Impairment: Do not use in patients with creatinine clearance <30 mL/min. (8.6)
- Hepatic Impairment: Do not use in patients with total bilirubin 1.5-3 × ULN and AST or ALT 2.5-10 × ULN, or total bilirubin > 3 × ULN. (8.7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Chronic Lymphocytic Leukemia (CLL)
1.2 Non-Hodgkin Lymphoma (NHL)
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Instructions for CLL
2.2 Dosing Instructions for NHL
2.3 Preparation for Intravenous Administration
2.4 Admixture Stability
2.5 Stability of Partially Used Vials (Needle Punched Vials)
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
5.2 Infections
5.3 Progressive Multifocal Leukoencephalopathy (PML)
5.4 Anaphylaxis and Infusion Reactions
5.5 Tumor Lysis Syndrome
5.6 Skin Reactions
5.7 Hepatotoxicity
5.8 Other Malignancies
5.9 Extravasation Injury
5.10 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on BENDEKA
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Chronic Lymphocytic Leukemia (CLL)
14.2 Non-Hodgkin Lymphoma (NHL)
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Instructions for CLL
Recommended Dosage:
The recommended dosage is 100 mg/m2 administered intravenously over 10 minutes on Days 1 and 2 of a 28-day cycle, up to 6 cycles.Dose Delays, Dosage Modifications and Reinitiation of Therapy for CLL:
Delay BENDEKA administration in the event of Grade 4 hematologic toxicity or clinically significant greater than or equal to Grade 2 non-hematologic toxicity. Once non-hematologic toxicity has recovered to less than or equal to Grade 1 and/or the blood counts have improved [Absolute Neutrophil Count (ANC) greater than or equal to 1 x 109/L, platelets greater than or equal to 75 x 109/L], reinitiate BENDEKA (bendamustine hydrochloride) injection at the discretion of the treating physician. In addition, consider dose reduction. [see Warnings and Precautions (5.1)]Dosage modifications for hematologic toxicity: for Grade 3 or greater toxicity, reduce the dose to 50 mg/m2 on Days 1 and 2 of each cycle; if Grade 3 or greater toxicity recurs, reduce the dose to 25 mg/m2 on Days 1 and 2 of each cycle.
Dosage modifications for non-hematologic toxicity: for clinically significant Grade 3 or greater toxicity, reduce the dose to 50 mg/m2 on Days 1 and 2 of each cycle.
Consider dosage re-escalation in subsequent cycles at the discretion of the treating physician.
2.2 Dosing Instructions for NHL
Recommended Dosage:
The recommended dose is 120 mg/m2 administered intravenously over 10 minutes on Days 1 and 2 of a 21-day cycle, up to 8 cycles.Dose Delays, Dosage Modifications and Reinitiation of Therapy for NHL:
Delay BENDEKA administration in the event of a Grade 4 hematologic toxicity or clinically significant greater than or equal to Grade 2 non-hematologic toxicity. Once non-hematologic toxicity has recovered to less than or equal to Grade 1 and/or the blood counts have improved [Absolute Neutrophil Count (ANC) greater than or equal to 1 x 109/L, platelets greater than or equal to 75 x 109/L], reinitiate BENDEKA at the discretion of the treating physician. In addition, consider dose reduction. [see Warnings and Precautions (5.1)]Dosage modifications for hematologic toxicity: for Grade 4 toxicity, reduce the dose to 90 mg/m2 on Days 1 and 2 of each cycle; if Grade 4 toxicity recurs, reduce the dose to 60 mg/m2 on Days 1 and 2 of each cycle.
Dosage modifications for non-hematologic toxicity: for Grade 3 or greater toxicity, reduce the dose to 90 mg/m2 on Days 1 and 2 of each cycle; if Grade 3 or greater toxicity recurs, reduce the dose to 60 mg/m2 on Days 1 and 2 of each cycle.
2.3 Preparation for Intravenous Administration
BENDEKA is a hazardous drug. Follow applicable special handling and disposal procedures.1
BENDEKA is in a multiple-dose vial. At room temperature, BENDEKA is a clear, and colorless to yellow ready-to-dilute solution. Store BENDEKA at recommended refrigerated storage conditions (2°C to 8°C or 36°F to 46°F). When refrigerated, the contents may freeze. Allow the vial to reach room temperature (15°C to 30°C or 59°F to 86°F) prior to use. Do not use the product if particulate matter is observed after achieving room temperature.
Intravenous Infusion
- Aseptically withdraw the volume needed for the required dose from the 25 mg/mL solution as per Table A below and immediately transfer the solution to a 50 mL infusion bag of one of the following diluents:
- –
- 0.9% Sodium Chloride Injection, USP; or
- –
- 2.5% Dextrose/0.45% Sodium Chloride Injection, USP; or
- –
- 5% Dextrose Injection, USP.
The resulting final concentration of bendamustine hydrochloride in the infusion bag should be within 0.49 mg/mL to 5.6 mg/mL. After transferring, thoroughly mix the contents of the infusion bag. The admixture should be a clear, and colorless to yellow solution.
No other diluents have been shown to be compatible. The 5% Dextrose Injection, USP, offers a sodium-free method of administration for patients with certain medical conditions requiring restricted sodium intake.
Table A: Volume (mL) of BENDEKA required for dilution into 50 mL of 0.9% saline, or 0.45% saline/2.5% dextrose or 5% dextrose for a given dose (mg/m2) and Body Surface Area (m2) Body Surface Area (m2) Volume of BENDEKA to withdraw (mL) 120 mg/m2 100 mg/m2 90 mg/m2 60 mg/m2 50 mg/m2 25 mg/m2 1 4.8 4 3.6 2.4 2 1 1.1 5.3 4.4 4 2.6 2.2 1.1 1.2 5.8 4.8 4.3 2.9 2.4 1.2 1.3 6.2 5.2 4.7 3.1 2.6 1.3 1.4 6.7 5.6 5 3.4 2.8 1.4 1.5 7.2 6 5.4 3.6 3 1.5 1.6 7.7 6.4 5.8 3.8 3.2 1.6 1.7 8.2 6.8 6.1 4.1 3.4 1.7 1.8 8.6 7.2 6.5 4.3 3.6 1.8 1.9 9.1 7.6 6.8 4.6 3.8 1.9 2 9.6 8 7.2 4.8 4 2 2.1 10.1 8.4 7.6 5 4.2 2.1 2.2 10.6 8.8 7.9 5.3 4.4 2.2 2.3 11 9.2 8.3 5.5 4.6 2.3 2.4 11.5 9.6 8.6 5.8 4.8 2.4 2.5 12 10 9 6 5 2.5 2.6 12.5 10.4 9.4 6.2 5.2 2.6 2.7 13 10.8 9.7 6.5 5.4 2.7 2.8 13.4 11.2 10.1 6.7 5.6 2.8 2.9 13.9 11.6 10.4 7 5.8 2.9 3 14.4 12 10.8 7.2 6 3 Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Any unused solution should be discarded according to institutional procedures for antineoplastics.
2.4 Admixture Stability
BENDEKA contains no antimicrobial preservative. Prepare the admixture as close as possible to the time of patient administration.
If diluted with 0.9% Sodium Chloride Injection, USP, or 2.5% Dextrose/0.45% Sodium Chloride Injection, USP, the final admixture is stable for 24 hours when stored refrigerated (2°C to 8°C or 36°F to 46°F) or for 6 hours when stored at room temperature (15°C to 30°C or 59°F to 86°F) and room light. Administration of diluted BENDEKA (bendamustine hydrochloride) injection must be completed within this period of time.
In the event that 5% Dextrose Injection, USP is utilized, the final admixture is stable for 24 hours when stored refrigerated (2°C to 8°C or 36°F to 46°F) or for only 3 hours when stored at room temperature (15°C to 30°C or 59°F to 86°F) and room light. Administration of diluted BENDEKA must be completed within this period of time.
Retain the partially used vial in original package to protect from light and store refrigerated (2°C to 8°C or 36°F to 46°F) if additional dose withdrawal from the same vial is intended.
2.5 Stability of Partially Used Vials (Needle Punched Vials)
BENDEKA is supplied in a multiple-dose vial. Although it does not contain any antimicrobial preservative, BENDEKA is bacteriostatic. The partially used vials are stable for up to 28 days when stored in its original carton under refrigeration (2°C to 8°C or 36°C to 46°F). Each vial is not recommended for more than a total of six (6) dose withdrawals.
After first use, store the partially used vial in the refrigerator in the original carton at 2°C to 8°C or 36°F to 46°F and then discard after 28 days.
- Aseptically withdraw the volume needed for the required dose from the 25 mg/mL solution as per Table A below and immediately transfer the solution to a 50 mL infusion bag of one of the following diluents:
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
BENDEKA is contraindicated in patients with a known hypersensitivity (e.g., anaphylactic and anaphylactoid reactions) to bendamustine, polyethylene glycol 400, propylene glycol, or monothioglycerol. [see Warnings and Precautions (5.4)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
Bendamustine hydrochloride caused severe myelosuppression (Grade 3-4) in 98% of patients in the two NHL studies [see Adverse Reactions (6.1)]. Three patients (2%) died from myelosuppression-related adverse reactions; one each from neutropenic sepsis, diffuse alveolar hemorrhage with Grade 3 thrombocytopenia, and pneumonia from an opportunistic infection (CMV).
BENDEKA causes myelosuppression. Monitor complete blood counts, including leukocytes, platelets, hemoglobin (Hgb), and neutrophils frequently. In the clinical trials, blood counts were monitored every week initially. Hematologic nadirs occurred predominantly in the third week of therapy. Myelosuppression may require dose delays and/or subsequent dose reductions if recovery to the recommended values has not occurred by the first day of the next scheduled cycle. Prior to the initiation of the next cycle of therapy, the ANC should be ≥ 1 x 109/L and the platelet count should be ≥ 75 x 109/L. [see Dosage and Administration (2.1)]
5.2 Infections
Infection, including pneumonia, sepsis, septic shock, hepatitis and death has occurred in adult and pediatric patients in clinical trials and in postmarketing reports for bendamustine hydrochloride [see Adverse Reactions (6.1, 6.2)]. Patients with myelosuppression following treatment with bendamustine hydrochloride are more susceptible to infections. Advise patients with myelosuppression following BENDEKA treatment to contact a physician immediately if they have symptoms or signs of infection.
Patients treated with BENDEKA are at risk for reactivation of infections including (but not limited to) hepatitis B, cytomegalovirus, Mycobacterium tuberculosis, and herpes zoster. Patients should undergo appropriate measures (including clinical and laboratory monitoring, prophylaxis, and treatment) for infection and infection reactivation prior to administration.
5.3 Progressive Multifocal Leukoencephalopathy (PML)
Progressive multifocal leukoencephalopathy (PML), including fatal cases, have occurred following treatment with bendamustine, primarily in combination with rituximab or obinutuzumab [see Adverse Reactions (6.2)]. Consider PML in the differential diagnosis in patients with new or worsening neurological, cognitive or behavioral signs or symptoms. If PML is suspected, withhold BENDEKA treatment and perform appropriate diagnostic evaluations. Consider discontinuation or reduction of any concomitant chemotherapy or immunosuppressive therapy in patients who develop PML.
5.4 Anaphylaxis and Infusion Reactions
Infusion reactions to bendamustine hydrochloride have occurred commonly in clinical trials [see Adverse Reactions (6.1)]. Symptoms include fever, chills, pruritus and rash. In rare instances, severe anaphylactic and anaphylactoid reactions have occurred, particularly in the second and subsequent cycles of therapy. Monitor clinically and discontinue drug for severe reactions. Ask patients about symptoms suggestive of infusion reactions after their first cycle of therapy. Patients who experienced Grade 3 or worse allergic-type reactions were not typically rechallenged. Consider measures to prevent severe reactions, including antihistamines, antipyretics and corticosteroids in subsequent cycles in patients who have experienced Grade 1 or 2 infusion reactions. Discontinue BENDEKA for patients with Grade 4 infusion reactions. Consider discontinuation for Grade 3 infusion reactions as clinically appropriate considering individual benefits, risks, and supportive care.
5.5 Tumor Lysis Syndrome
Tumor lysis syndrome associated with bendamustine hydrochloride has occurred in patients in clinical trials and in postmarketing reports [see Adverse Reactions (6.1)]. The onset tends to be within the first treatment cycle of bendamustine hydrochloride and, without intervention, may lead to acute renal failure and death. Preventive measures include vigorous hydration and close monitoring of blood chemistry, particularly potassium and uric acid levels. Allopurinol has also been used during the beginning of bendamustine hydrochloride therapy. However, there may be an increased risk of severe skin toxicity when bendamustine hydrochloride and allopurinol are administered concomitantly. [see Warnings and Precautions (5.6)]
5.6 Skin Reactions
Fatal and serious skin reactions have been reported with bendamustine hydrochloride injection treatment in clinical trials and postmarketing safety reports, including toxic skin reactions [Stevens-Johnson Syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS)], bullous exanthema, and rash [see Adverse Reactions (6.1 and 6.2)]. Events occurred when bendamustine hydrochloride injection was given as a single agent and in combination with other anticancer agents or allopurinol.
Where skin reactions occur, they may be progressive and increase in severity with further treatment. Monitor patients with skin reactions closely. If skin reactions are severe or progressive, withhold or discontinue BENDEKA.
5.7 Hepatotoxicity
Fatal and serious cases of liver injury have been reported with bendamustine hydrochloride injection [see Adverse Reactions (6.1)]. Combination therapy, progressive disease or reactivation of hepatitis B were confounding factors in some patients [see Warnings and Precautions (5.2)]. Most cases were reported within the first three months of starting therapy. Monitor liver chemistry tests prior to and during BENDEKA therapy.
5.8 Other Malignancies
There are reports of pre-malignant and malignant diseases that have developed in patients who have been treated with bendamustine hydrochloride, including myelodysplastic syndrome, myeloproliferative disorders, acute myeloid leukemia, bronchial carcinoma, and non-melanoma skin cancer, including basal cell carcinoma and squamous cell carcinoma [see Adverse Reactions (6.2)].
Monitor patients for the development of secondary malignancies. Perform dermatologic evaluations during and after treatment with BENDEKA.
5.9 Extravasation Injury
Bendamustine hydrochloride extravasations have been reported in postmarketing resulting in hospitalizations from erythema, marked swelling, and pain [see Adverse Reactions (6.2)]. Assure good venous access prior to starting drug infusion and monitor the intravenous infusion site for redness, swelling, pain, infection, and necrosis during and after administration of BENDEKA.
5.10 Embryo-Fetal Toxicity
Based on findings from animal reproduction studies and the drug’s mechanism of action, BENDEKA can cause fetal harm when administered to a pregnant woman. Single intraperitoneal doses of bendamustine (that approximated the maximum recommended human dose based on body surface area) to pregnant mice and rats during organogenesis caused adverse developmental outcomes, including an increase in resorptions, skeletal and visceral malformations, and decreased fetal body weights. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use an effective method of contraception during treatment with BENDEKA and for 6 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with BENDEKA and for 3 months after the last dose. [see ( Use in Specific Populations 8.1, 8.3) and Clinical Pharmacology (12.1)]
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions have been associated with bendamustine hydrochloride in clinical trials and are discussed in greater detail in other sections of the prescribing information.
- Myelosuppression [see Warnings and Precautions (5.1)]
- Infections [see Warnings and Precautions (5.2)]
- Progressive Multifocal Leukoencephalopathy [see Warnings and Precautions (5.3)]
- Anaphylaxis and Infusion Reactions[see Warnings and Precautions (5.4)]
- Tumor Lysis Syndrome [see Warnings and Precautions (5.5)]
- Skin Reactions [see Warnings and Precautions (5.6)]
- Hepatotoxicity [see Warnings and Precautions (5.7)]
- Other Malignancies [see Warnings and Precautions (5.8)]
- Extravasation Injury [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to bendamustine hydrochloride in 329 patients who participated in an actively controlled trial (N=153) for the treatment of CLL and two single arm studies (N=176) for the treatment of indolent B cell NHL. Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of BENDEKA administered IV as a 50 mL admixture over a 10-minute infusion is supported by clinical trials using bendamustine hydrochloride administered IV as a 500 mL admixture over 30-60 minutes infusion time, as well as an open-label, crossover study in 81 ‘end-of-life’ cancer patients treated with BENDEKA. In total, safety data from clinical studies are available from over 400 cancer patients exposed to bendamustine hydrochloride at doses in the range used in the treatment of CLL and NHL.
No clinically significant differences in the adverse reaction profile were noted among bendamustine hydrochloride administered as a 500 mL admixture over standard infusion time (30-60 minutes) and BENDEKA administered as a 50 mL admixture in a ‘short-time’ infusion over 10 minutes.
The safety and tolerability of BENDEKA was evaluated in an 8-week clinical study of BENDEKA in 81 ‘end-of-life’ cancer patients, diagnosed with solid tumors and hematologic malignancies (excluding CLL). The population was 40-82 years of age, 58% females, 84% white, 12.3% Black, 1.2% Asian and 2.5% were classified as ‘other’. BENDEKA was administered IV at a 120 mg/m2 dose as a 50 mL admixture over 10 minutes. Patients in the study received BENDEKA (50 mL IV, over 10 minutes) or bendamustine hydrochloride (500 mL IV, over 60 minutes) on Days 1 and 2 every 28 days for two consecutive 2-day cycles.
Adverse reactions (any grade) that occurred with a frequency greater than 5% during BENDEKA infusion and within one hour post-infusion were nausea (8.2%) and fatigue (5.5%).
Adverse reactions (any grade) that occurred with a frequency greater than 5% within 24 hours of BENDEKA were nausea (10.9%) and fatigue (8.2%).
Adverse reactions leading to study withdrawal in 4 patients receiving BENDEKA were pyrexia (1.2%), nausea (1.2%), vomiting (1.2%), pneumonia (1.2%) and fatigue (1.2%).
Chronic Lymphocytic Leukemia (CLL)
The data described below reflect exposure to bendamustine hydrochloride in 153 patients. Bendamustine hydrochloride was studied in an active-controlled randomized trial. The population was 45-77 years of age, 63% male, 100% white, and had treatment naïve CLL. All patients started the study at a dose of 100 mg/m2 intravenously over 30 minutes on Days 1 and 2 every 28 days.Adverse reactions were reported according to NCI CTC v.2.0. In the randomized CLL clinical study, non-hematologic adverse reactions (any grade) in the bendamustine hydrochloride group that occurred with a frequency greater than 15% were pyrexia (24%), nausea (20%), and vomiting (16%).
Other adverse reactions seen frequently in one or more studies included asthenia, fatigue, malaise, and weakness; dry mouth; somnolence; cough; constipation; headache; mucosal inflammation and stomatitis.
Worsening hypertension was reported in 4 patients treated with bendamustine hydrochloride in the randomized CLL clinical study and in none treated with chlorambucil. Three of these 4 adverse reactions were described as a hypertensive crisis and were managed with oral medications and resolved.
The most frequent adverse reactions leading to study withdrawal for patients receiving bendamustine hydrochloride were hypersensitivity (2%) and pyrexia (1%).
Table 1 contains the treatment emergent adverse reactions, regardless of attribution, that were reported in ≥ 5% of patients in either treatment group in the randomized CLL clinical study.
Table 1: Non-Hematologic Adverse Reactions Occurring in Randomized CLL Clinical Study in at Least 5% of Patients Number (%) of patients Bendamustine Hydrochloride
(N=153)Chlorambucil
(N=143)Body System
Adverse ReactionAll Grades Grade 3/4 All Grades Grade 3/4 Total number of patients
with at least 1 adverse
reaction121 (79) 52 (34) 96 (67) 25 (17) Gastrointestinal disorders Nausea 31 (20) 1 (<1) 21 (15) 1 (<1) Vomiting 24 (16) 1 (<1) 9 (6) 0 Diarrhea 14 (9) 2 (1) 5 (3) 0 General disorders and
administration site
conditionsPyrexia 36 (24) 6 (4) 8 (6) 2 (1) Fatigue 14 (9) 2 (1) 8 (6) 0 Asthenia 13 (8) 0 6 (4) 0 Chills 9 (6) 0 1 (<1) 0 Immune system disorders Hypersensitivity 7 (5) 2 (1) 3 (2) 0 Infections and infestations Nasopharyngitis 10 (7) 0 12 (8) 0 Infection 9 (6) 3 (2) 1 (<1) 1 (<1) Herpes simplex 5 (3) 0 7 (5) 0 Investigations Weight decreased 11 (7) 0 5 (3) 0 Metabolism and nutrition disorders Hyperuricemia 11 (7) 3 (2) 2 (1) 0 Respiratory, thoracic and mediastinal disorders Cough 6 (4) 1 (<1) 7 (5) 1 (<1) Skin and subcutaneous tissue disorders Rash 12 (8) 4 (3) 7 (5) 3 (2) Pruritus 8 (5) 0 2 (1) 0 The Grade 3 and 4 hematology laboratory test values by treatment group in the randomized CLL clinical study are described in Table 2. These findings confirm the myelosuppressive effects seen in patients treated with bendamustine hydrochloride. Red blood cell transfusions were administered to 20% of patients receiving bendamustine hydrochloride compared with 6% of patients receiving chlorambucil.
Table 2: Incidence of Hematology Laboratory Abnormalities in Patients Who Received bendamustine hydrochloride or Chlorambucil in the Randomized CLL Clinical Study Laboratory
AbnormalityBendamustine Hydrochloride
(N=150)Chlorambucil
(N=141)All Grades
n (%)Grade 3/4
n (%)All Grades
n (%)Grade 3/4
n (%)Hemoglobin
Decreased134 (89) 20 (13) 115 (82) 12 (9) Platelets
Decreased116 (77) 16 (11) 110 (78) 14 (10) Leukocytes
Decreased92 (61) 42 (28) 26 (18) 4 (3) Lymphocytes
Decreased102 (68) 70 (47) 27 (19) 6 (4) Neutrophils
Decreased113 (75) 65 (43) 86 (61) 30 (21) In the randomized CLL trial, 34% of patients had bilirubin elevations, some without associated significant elevations in AST and ALT. Grade 3 or 4 increased bilirubin occurred in 3% of patients. Increases in AST and ALT of Grade 3 or 4 were limited to 1% and 3% of patients, respectively. Patients treated with bendamustine hydrochloride may also have changes in their creatinine levels. If abnormalities are detected, monitoring of these parameters should be continued to ensure that significant deterioration does not occur.
Non-Hodgkin’s Lymphoma (NHL)
The data described below reflect exposure to bendamustine hydrochloride in 176 patients with indolent B-cell NHL treated in two single-arm studies. The population was 31-84 years of age, 60% male, and 40% female. The race distribution was 89% White, 7% Black, 3% Hispanic, 1% other, and <1% Asian. These patients received bendamustine hydrochloride at a dose of 120 mg/m2 intravenously on Days 1 and 2 for up to eight 21-day cycles.The adverse reactions occurring in at least 5% of the NHL patients, regardless of severity, are shown in Table 3. The most common non-hematologic adverse reactions (≥30%) were nausea (75%), fatigue (57%), vomiting (40%), diarrhea (37%) and pyrexia (34%). The most common non-hematologic Grade 3 or 4 adverse reactions (≥5%) were fatigue (11%), febrile neutropenia (6%), and pneumonia, hypokalemia and dehydration, each reported in 5% of patients.
Table 3: Non-Hematologic Adverse Reactions Occurring in at Least 5% of NHL Patients Treated with bendamustine hydrochloride by System Organ Class and Preferred Term (N=176) *Patients may have reported more than 1 adverse reaction.
NOTE: Patients counted only once in each preferred term category and once in each system organ class category.
Body System Number (%) of patients* Adverse Reaction All Grades Grade 3/4 Total number of patients with at
least 1 adverse reaction176 (100) 94 (53) Cardiac Disorders Tachycardia 13 (7) 0 Gastrointestinal disorders Nausea 132 (75) 7 (4) Vomiting 71 (40) 5 (3) Diarrhea 65 (37) 6 (3) Constipation 51 (29) 1 (<1) Stomatitis 27 (15) 1 (<1) Abdominal pain 22 (13) 2 (1) Dyspepsia 20 (11) 0 Gastroesophageal reflux disease 18 (10) 0 Dry mouth 15 (9) 1 (<1) Abdominal pain upper 8 (5) 0 Abdominal distension 8 (5) 0 General disorders and administration site conditions Fatigue 101 (57) 19 (11) Pyrexia 59 (34) 3 (2) Chills 24 (14) 0 Edema peripheral 23 (13) 1 (<1) Asthenia 19 (11) 4 (2) Chest pain 11 (6) 1 (<1) Infusion site pain 11 (6) 0 Pain 10 (6) 0 Catheter site pain 8 (5) 0 Infections and infestations Herpes zoster 18 (10) 5 (3) Upper respiratory tract infection 18 (10) 0 Urinary tract infection 17 (10) 4 (2) Sinusitis 15 (9) 0 Pneumonia 14 (8) 9 (5) Febrile neutropenia 11 (6) 11 (6) Oral candidiasis 11 (6) 2 (1) Nasopharyngitis 11 (6) 0 Investigations Weight decreased 31 (18) 3 (2) Metabolism and nutrition disorders Anorexia 40 (23) 3 (2) Dehydration 24 (14) 8 (5) Decreased appetite 22 (13) 1 (<1) Hypokalemia 15 (9) 9 (5) Musculoskeletal and connective tissue disorders Back pain 25 (14) 5 (3) Arthralgia 11 (6) 0 Pain in extremity 8 (5) 2 (1) Bone pain 8 (5) 0 Nervous system disorders Headache 36 (21) 0 Dizziness 25 (14) 0 Dysgeusia 13 (7) 0 Psychiatric disorder Insomnia 23 (13) 0 Anxiety 14 (8) 1 (<1) Depression 10 (6) 0 Respiratory, thoracic and mediastinal disorders Cough 38 (22) 1 (<1) Dyspnea 28 (16) 3 (2) Pharyngolaryngeal pain 14 (8) 1 (<1) Wheezing 8 (5) 0 Nasal congestion 8 (5) 0 Skin and subcutaneous tissue disorders Rash 28 (16) 1 (<1) Pruritus 11 (6) 0 Dry skin 9 (5) 0 Night sweats 9 (5) 0 Hyperhidrosis 8 (5) 0 Vascular disorders Hypotension 10 (6) 2 (1) Hematologic toxicities, based on laboratory values and CTC grade, in NHL patients treated in both single arm studies combined are described in Table 4. Clinically important chemistry laboratory values that were new or worsened from baseline and occurred in >1% of patients at grade 3 or 4, in NHL patients treated in both single arm studies combined were hyperglycemia (3%), elevated creatinine (2%), hyponatremia (2%), and hypocalcemia (2%).
Table 4: Incidence of Hematology Laboratory Abnormalities in Patients Who Received bendamustine hydrochloride in the NHL Studies Hematology Variable Percent of Patients All Grades Grade 3/4 Lymphocytes Decreased 99 94 Leukocytes Decreased 94 56 Hemoglobin Decreased 88 11 Neutrophils Decreased 86 60 Platelets Decreased 86 25 In both studies, serious adverse reactions, regardless of causality, were reported in 37% of patients receiving bendamustine hydrochloride. The most common serious adverse reactions occurring in ≥5% of patients were febrile neutropenia and pneumonia. Other important serious adverse reactions reported in clinical trials and/or postmarketing experience were acute renal failure, cardiac failure, hypersensitivity, skin reactions, pulmonary fibrosis, and myelodysplastic syndrome.
Serious drug-related adverse reactions reported in clinical trials included myelosuppression, infection, pneumonia, tumor lysis syndrome and infusion reactions. Adverse reactions occurring less frequently but possibly related to bendamustine hydrochloride treatment were hemolysis, dysgeusia/taste disorder, atypical pneumonia, sepsis, herpes zoster, erythema, dermatitis, and skin necrosis.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of bendamustine hydrochloride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic systems disorders: Pancytopenia.
Cardiovascular disorders: Atrial fibrillation, congestive heart failure (some fatal), myocardial infarction (some fatal), palpitation.
General disorders and administration site conditions: Injection site reactions (including phlebitis, pruritus, irritation, pain, swelling), infusion site reactions (including phlebitis, pruritus, irritation, pain, swelling).
Immune system disorders: Anaphylaxis.
Infections and infestations: Pneumocystis jirovecii pneumonia, progressive multifocal leukoencephalopathy (PML).
Renal and urinary disorders: Nephrogenic diabetes insipidus (NDI).
Respiratory, thoracic and mediastinal disorders: Pneumonitis.
Skin and subcutaneous tissue disorders: Drug reaction with eosinophilia and systemic symptoms (DRESS), non-melanoma skin cancer (NMSC), Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN).
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on BENDEKA
CYP1A2 Inhibitors
The coadministration of BENDEKA with CYP1A2 inhibitors may increase bendamustine plasma concentrations and may result in increased incidence of adverse reactions with BENDEKA [see Clinical Pharmacology (12.3)]. Consider alternative therapies that are not CYP1A2 inhibitors during treatment with BENDEKA.CYP1A2 Inducers
The coadministration of BENDEKA with CYP1A2 inducers may decrease bendamustine plasma concentrations and may result in decreased efficacy of BENDEKA [see Clinical Pharmacology (12.3)]. Consider alternative therapies that are not CYP1A2 inducers during treatment with BENDEKA. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
In animal reproduction studies, intraperitoneal administration of bendamustine to pregnant mice and rats during organogenesis at doses 0.6 to 1.8 times the maximum recommended human dose (MRHD) resulted in embryo-fetal and/or infant mortality, structural abnormalities, and alterations to growth (see Data). There are no available data on bendamustine hydrochloride use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Advise pregnant women of the potential risk to a fetus.The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Bendamustine hydrochloride was intraperitoneally administered once to mice from 210 mg/m2 (approximately 1.8 times the MRHD) during organogenesis and caused an increase in resorptions, skeletal and visceral malformations (exencephaly, cleft palates, accessory rib, and spinal deformities) and decreased fetal body weights. This dose did not appear to be maternally toxic and lower doses were not evaluated. Repeat intraperitoneal administration of bendamustine hydrochloride to mice on gestation days 7 to 11 resulted in an increase in resorptions from 75 mg/m2 (approximately 0.6 times the MRHD) and an increase in abnormalities from 112.5 mg/m2 (approximately 0.9 times the MRHD), similar to those seen after a single intraperitoneal administration.Bendamustine hydrochloride was intraperitoneally administered once to rats from 120 mg/m2 (approximately the MRHD) on gestation days 4, 7, 9, 11, or 13 and caused embryo and fetal lethality as indicated by increased resorptions and a decrease in live fetuses. A significant increase in external (effect on tail, head, and herniation of external organs [exomphalos]) and internal (hydronephrosis and hydrocephalus) malformations were seen in dosed rats.
8.2 Lactation
Risk Summary
There are no data on the presence of bendamustine hydrochloride or its metabolites in either human or animal milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in the breastfed child, advise patients that breastfeeding is not recommended during treatment with BENDEKA, and for 1 week after the last dose.8.3 Females and Males of Reproductive Potential
BENDEKA can cause embryo-fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Pregnancy testing is recommended for females of reproductive potential prior to initiation of BENDEKA [see Use in Specific Populations (8.1)].Contraception
Females
Advise female patients of reproductive potential to use effective contraception during treatment with BENDEKA and for 6 months after the last dose.Males
Based on genotoxicity findings, advise males with female partners of reproductive potential to use effective contraception during treatment with BENDEKA and for 3 months after the last dose [see Nonclinical Toxicology (13.1)].Infertility
Males
Based on findings from clinical studies, BENDEKA may impair male fertility. Impaired spermatogenesis, azoospermia, and total germinal aplasia have been reported in male patients treated with alkylating agents, especially in combination with other drugs. In some instances, spermatogenesis may return in patients in remission, but this may occur only several years after intensive chemotherapy has been discontinued. Advise patients of the potential risk to their reproductive capacities.Based on findings from animal studies, BENDEKA may impair male fertility due to an increase in morphologically abnormal spermatozoa. The long-term effects of BENDEKA on male fertility, including the reversibility of adverse effects, have not been studied [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Safety, pharmacokinetics and efficacy were assessed in a single open-label trial (NCT01088984) in patients aged 1 to 19 years with relapsed or refractory acute leukemia, including 27 patients with acute lymphocytic leukemia (ALL) and 16 patients with acute myeloid leukemia (AML). Bendamustine hydrochloride was administered as an intravenous infusion over 60 minutes on Days 1 and 2 of each 21-day cycle. There was no treatment response (CR+ CRp) in any patient. The safety profile in these patients was consistent with that seen in adults, and no new safety signals were identified.
The pharmacokinetics of bendamustine in 43 patients, aged 1 to 19 years (median age of 10 years) were within range of values previously observed in adults given the same dose based on body surface area.
8.5 Geriatric Use
No overall differences in safety were observed between patients ≥65 years of age and younger patients. Efficacy was lower in patients 65 and over with CLL receiving bendamustine hydrochloride based upon an overall response rate of 47% for patients 65 and over and 70% for younger patients. Progression free survival was also longer in younger patients with CLL receiving bendamustine (19 months vs. 12 months). No overall differences in efficacy in patients with non-Hodgkin Lymphoma were observed between geriatric patients and younger patients.
8.6 Renal Impairment
Do not use BENDEKA in patients with creatinine clearance (CLcr) < 30 mL/min [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Do not use BENDEKA in patients with AST or ALT 2.5-10 × upper limit of normal (ULN) and total bilirubin 1.5-3 × ULN, or total bilirubin > 3 × ULN [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
The intravenous LD50 of bendamustine hydrochloride is 240 mg/m2 in the mouse and rat. Toxicities included sedation, tremor, ataxia, convulsions and respiratory distress.
Across all clinical experience, the reported maximum single dose received was 280 mg/m2. Three of four patients treated at this dose showed ECG changes considered dose-limiting at 7 and 21 days post-dosing. These changes included QT prolongation (one patient), sinus tachycardia (one patient), ST and T wave deviations (two patients) and left anterior fascicular block (one patient). Cardiac enzymes and ejection fractions remained normal in all patients.
No specific antidote for bendamustine hydrochloride overdose is known. Management of overdosage should include general supportive measures, including monitoring of hematologic parameters and ECGs.
-
11 DESCRIPTION
BENDEKA (bendamustine hydrochloride) injection is an alkylating agent. The chemical name of bendamustine hydrochloride is 1H-benzimidazole-2-butanoic acid, 5-[bis(2-chloroethyl)amino]-1 methyl-, monohydrochloride. Its empirical molecular formula is C16H21Cl2N3O2 · HCl, and the molecular weight is 394.7. Bendamustine hydrochloride contains a mechlorethamine group and a benzimidazole heterocyclic ring with a butyric acid substituent, and has the following structural formula:
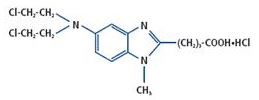
BENDEKA (bendamustine hydrochloride) injection for intravenous use is supplied as a sterile, clear, and colorless to yellow ready-to-dilute solution in a multiple-dose clear glass vial. Each milliliter contains 25 mg of bendamustine hydrochloride, USP, 0.1 mL of Propylene Glycol, USP, 5 mg of Monothioglycerol, NF, in Polyethylene Glycol 400, NF. Sodium hydroxide may have been used to adjust the acidity of polyethylene glycol 400.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Bendamustine is a bifunctional mechlorethamine derivative containing a purine-like benzimidazole ring. Mechlorethamine and its derivatives form electrophilic alkyl groups. These groups form covalent bonds with electron-rich nucleophilic moieties, resulting in interstrand DNA crosslinks. The bifunctional covalent linkage can lead to cell death via several pathways. Bendamustine is active against both quiescent and dividing cells. The exact mechanism of action of bendamustine remains unknown.
12.2 Pharmacodynamics
Based on the pharmacokinetics/pharmacodynamics analyses of data from adult patients with NHL, nausea increased with increasing bendamustine Cmax.
Cardiac Electrophysiology
The effect of bendamustine on the QTc interval was evaluated in 53 patients with indolent NHL and mantle cell lymphoma on Day 1 of Cycle 1 after administration of rituximab at 375 mg/m2 intravenous infusion followed by a 30-minute intravenous infusion of bendamustine at 90 mg/m2/day. No mean changes greater than 20 milliseconds were detected up to one hour post infusion. The potential for delayed effects on the QT interval after one hour was not evaluated.12.3 Pharmacokinetics
Following a single dose of 120 mg/m2 of bendamustine hydrochloride over 10-minute infusion, the mean Cmax achieved was 35 µg/mL (range 6 to 49 µg/mL), occurring typically at the end of infusion. Little or no accumulation in plasma is expected for bendamustine administered on Days 1 and 2 of a 28-day cycle.
Distribution
The protein binding of bendamustine ranged from 94 to 96% and was concentration independent from 1 to 50 µg/mL. The blood to plasma concentration ratios in human blood ranged from 0.84 to 0.86 over a concentration range of 10 to 100 µg/mL.The mean steady-state volume of distribution (VSS) of bendamustine was approximately 20 to 25 L.
Elimination
After a single intravenous dose of 120 mg/m2 of bendamustine over 1 hour, the intermediate half-life (t½) of the parent compound is approximately 40 minutes. The mean terminal elimination t½ of two active metabolites, γ-hydroxybendamustine (M3) and N desmethylbendamustine (M4) are approximately 3 hours and 30 minutes, respectively. Bendamustine clearance in humans is approximately 700 mL/min.Metabolism
Bendamustine is extensively metabolized via hydrolytic, oxidative, and conjugative pathways. Bendamustine is primarily metabolized via hydrolysis to monohydroxy (HP1) and dihydroxybendamustine (HP2) metabolites with low cytotoxic activity in vitro. Two active minor metabolites, M3 and M4, are primarily formed via CYP1A2 in vitro. M3 and M4 concentrations of these metabolites in plasma are 1/10th and 1/100th that of the parent compound, respectively.Excretion
Following IV infusion of radiolabeled bendamustine hydrochloride in cancer patients, approximately 76% of the dose was recovered. Approximately 50% of the dose was recovered in the urine (3.3% unchanged) and approximately 25% of the dose was recovered in the feces. Less than 1% of the dose was recovered in the urine as M3 and M4, and less than 5% of the dose was recovered in the urine as HP2.Specific Populations
No clinically meaningful effects on the pharmacokinetics of bendamustine were observed based on age (31 to 84 years), sex, mild to moderate renal impairment (CLcr ≥ 30 mL/min), or hepatic impairment with total bilirubin 1.5 < ULN and AST or ALT < 2.5 × ULN. The effects of severe renal impairment (CLcr < 30 mL/min), or hepatic impairment with total bilirubin 1.5-3 × ULN and AST or ALT 2.5-10 × ULN or total bilirubin > 3 × ULN on the pharmacokinetics of bendamustine is unknown.Race/Ethnicity
Exposures in Japanese subjects (n=6) were 40% higher than that in non-Japanese subjects receiving the same dose. The clinical importance of this difference on the safety and efficacy of bendamustine hydrochloride in Japanese subjects has not been established.Drug Interaction Studies
In Vitro Studies
Effect of Bendamustine on CYP Substrates
Bendamustine did not inhibit CYP1A2, 2C9/10, 2D6, 2E1, or 3A4/5. Bendamustine did not induce metabolism of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2E1, or CYP3A4/5.Effect of Transporters on Bendamustine Hydrochloride
Bendamustine is a substrate of P-glycoprotein and breast cancer resistance protein (BCRP). -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Bendamustine was carcinogenic in mice. After intraperitoneal injections at 37.5 mg/m2/day (the lowest dose tested, approximately 0.3 times the maximum recommended human dose [MRHD]) and 75 mg/m2/day (approximately 0.6 times the MRHD) for 4 days, peritoneal sarcomas in female AB/Jena mice were produced. Oral administration at 187.5 mg/m2/day (the only dose tested, approximately 1.6 times the MRHD) for 4 days induced mammary carcinomas and pulmonary adenomas.
Bendamustine is a mutagen and clastogen. In a bacterial reverse mutation assay (Ames assay), bendamustine was shown to increase revertant frequency in the absence and presence of metabolic activation. Bendamustine was clastogenic in human lymphocytes in vitro, and in rat bone marrow cells in vivo (increase in micronucleated polychromatic erythrocytes) from 37.5 mg/m2, (the lowest dose tested, approximately 0.3 times the MRHD).
Bendamustine induced morphologic abnormalities in spermatozoa in mice. Following tail vein injection of bendamustine at 120 mg/m2 or a saline control on days 1 and 2 for a total of 3 weeks, the number of spermatozoa with morphologic abnormalities was 16% higher in the bendamustine-treated group as compared to the saline control group.
-
14 CLINICAL STUDIES
14.1 Chronic Lymphocytic Leukemia (CLL)
The safety and efficacy of bendamustine hydrochloride were evaluated in an open-label, randomized, controlled multicenter trial comparing bendamustine hydrochloride to chlorambucil. The trial was conducted in 301 previously-untreated patients with Binet Stage B or C (Rai Stages I - IV) CLL requiring treatment. Need-to-treat criteria included hematopoietic insufficiency, B-symptoms, rapidly progressive disease or risk of complications from bulky lymphadenopathy. Patients with autoimmune hemolytic anemia or autoimmune thrombocytopenia, Richter’s syndrome, or transformation to prolymphocytic leukemia were excluded from the study.
The patient populations in the bendamustine hydrochloride and chlorambucil treatment groups were balanced with regard to the following baseline characteristics: age (median 63 vs. 66 years), gender (63% vs. 61% male), Binet stage (71% vs. 69% Binet B), lymphadenopathy (79% vs. 82%), enlarged spleen (76% vs. 80%), enlarged liver (48% vs. 46%), hypercellular bone marrow (79% vs. 73%), “B” symptoms (51% vs. 53%), lymphocyte count (mean 65.7x109/L vs. 65.1x109/L), and serum lactate dehydrogenase concentration (mean 370.2 vs. 388.4 U/L). Ninety percent of patients in both treatment groups had immuno-phenotypic confirmation of CLL (CD5, CD23 and either CD19 or CD20 or both).
Patients were randomly assigned to receive either bendamustine hydrochloride at 100 mg/m2, administered intravenously over a period of 30 minutes on Days 1 and 2 or chlorambucil at 0.8 mg/kg (Broca’s normal weight) administered orally on Days 1 and 15 of each 28-day cycle. Efficacy endpoints of objective response rate and progression-free survival were calculated using a pre-specified algorithm based on NCI working group criteria for CLL.
The results of this open-label randomized study demonstrated a higher rate of overall response and a longer progression-free survival for bendamustine hydrochloride compared to chlorambucil (see Table 5). Survival data are not mature.
Table 5: Efficacy Data for CLL CI = confidence interval
* CR was defined as peripheral lymphocyte count ≤ 4 x 109/L, neutrophils ≥ 1.5 x 109/L, platelets >100 x 109/L, hemoglobin > 110g/L, without transfusions, absence of palpable hepatosplenomegaly, lymph nodes ≤ 1.5 cm, < 30% lymphocytes without nodularity in at least a normocellular bone marrow and absence of “B” symptoms. The clinical and laboratory criteria were required to be maintained for a period of at least 56 days.
** nPR was defined as described for CR with the exception that the bone marrow biopsy shows persistent nodules.
† PR was defined as ≥50% decrease in peripheral lymphocyte count from the pretreatment baseline value, and either ≥50% reduction in lymphadenopathy, or ≥50% reduction in the size of spleen or liver, as well as one of the following hematologic improvements: neutrophils ≥ 1.5 x 109/L or 50% improvement over baseline, platelets >100 x 109/L or 50% improvement over baseline, hemoglobin >110g/L or 50% improvement over baseline without transfusions, for a period of at least 56 days.
†† PFS was defined as time from randomization to progression or death from any cause.
Bendamustine Hydrochloride
(N=153)Chlorambucil
(N=148)p-value Response Rate n (%) Overall response rate 90 (59) 38 (26) <0.0001 (95% CI) (51, 66.6) (18.6, 32.7) Complete response (CR)* 13 (8) 1 (<1) Nodular partial response (nPR)** 4 (3) 0 Partial response (PR) † 73 (48) 37 (25) Progression-Free Survival†† Median, months (95% CI) 18 (11.7, 23.5) 6 (5.6, 8.6) Hazard ratio (95% CI) 0.27 (0.17, 0.43)
<0.0001 Kaplan-Meier estimates of progression-free survival comparing bendamustine hydrochloride with chlorambucil are shown in Figure 1.
Figure 1. Progression-Free Survival
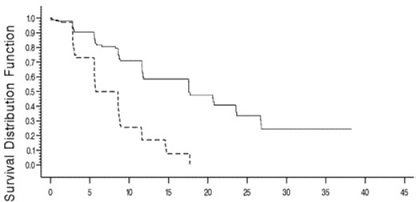
Progression – Free Survival (months) Study Treatment ____
Bendamustine Hydrochloride_ _ _
Chlorambucil14.2 Non-Hodgkin Lymphoma (NHL)
The efficacy of bendamustine hydrochloride was evaluated in a single arm study (NCT00139841) of 100 patients with indolent B-cell NHL that had progressed during or within six months of treatment with rituximab or a rituximab-containing regimen. Patients were included if they relapsed within 6 months of either the first dose (monotherapy) or last dose (maintenance regimen or combination therapy) of rituximab. All patients received bendamustine hydrochloride intravenously at a dose of 120 mg/m2, on Days 1 and 2 of a 21-day treatment cycle. Patients were treated for up to 8 cycles.
The median age was 60 years, 65% were male, and 95% had a baseline WHO performance status of 0 or 1. Major tumor subtypes were follicular lymphoma (62%), diffuse small lymphocytic lymphoma (21%), and marginal zone lymphoma (16%). Ninety-nine percent of patients had received previous chemotherapy, 91% of patients had received previous alkylator therapy, and 97% of patients had relapsed within 6 months of either the first dose (monotherapy) or last dose (maintenance regimen or combination therapy) of rituximab.
Efficacy was based on the assessments by a blinded independent review committee (IRC) and included overall response rate (complete response + complete response unconfirmed + partial response) and duration of response (DR) as summarized in Table 6.
Table 6: Efficacy Data for NHL* CI = confidence interval
*IRC assessment was based on modified International Working Group response criteria (IWG-RC). Modifications to IWG-RC specified that a persistently positive bone marrow in patients who met all other criteria for CR would be scored as PR. Bone marrow sample lengths were not required to be ≥20 mm.
Bendamustine Hydrochloride
(N=100)Response Rate (%) Overall response rate (CR+CRu+PR) 74 (95% CI) (64.3, 82.3) Complete response (CR) 13 Complete response unconfirmed (CRu) 4 Partial response (PR) 57 Duration of Response (DR) Median, months (95% CI) 9.2 months
(7.1, 10.8) - 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Safe Handling and Disposal
BENDEKA (bendamustine hydrochloride) injection is a hazardous drug. Follow applicable special handling and disposal procedures.1 Care should be exercised in the handling and preparation of solutions prepared from BENDEKA (bendamustine hydrochloride) injection. The use of gloves and safety glasses is recommended to avoid exposure in case of breakage of the vial or other accidental spillage. If a solution of BENDEKA (bendamustine hydrochloride) injection contacts the skin, wash the skin immediately and thoroughly with soap and water. If BENDEKA (bendamustine hydrochloride) injection contacts the mucous membranes, flush thoroughly with water.How Supplied
BENDEKA (bendamustine hydrochloride) injection is supplied in individual cartons of 5 mL clear multiple-dose vials containing 100 mg of bendamustine hydrochloride as a clear, and colorless to yellow ready-to-dilute solution.
- NDC 63459-348-04, 100 mg/4 mL (25 mg/mL)
Storage
Store BENDEKA (bendamustine hydrochloride) injection in refrigerator, 2°C to 8°C (36°F to 46°F). Retain in original carton until time of use to protect from light. -
17 PATIENT COUNSELING INFORMATION
Myelosuppression
Inform patients of the likelihood that BENDEKA (bendamustine hydrochloride) injection will cause a decrease in white blood cells, platelets, and red blood cells. They will need frequent monitoring of these parameters. They should be instructed to report shortness of breath, significant fatigue, bleeding, fever, or other signs of infection [see Warnings and Precautions (5.1)].Progressive Multifocal Leukoencephalopathy (PML)
Inform patients to immediately contact their healthcare provider if they experience confusion, memory loss, trouble thinking, difficulty talking or walking, vision loss or other neurological or cognitive symptoms [see Warnings and Precautions (5.3)].Anaphylaxis and Infusion Reactions
Inform patients of the possibility of serious or mild allergic reactions and to immediately report rash, facial swelling, or difficulty breathing during or soon after infusion [see Warnings and Precautions (5.4)].Skin Reactions
Advise patients that a mild rash or itching may occur during treatment with BENDEKA (bendamustine hydrochloride) injection. Advise patients to immediately report severe or worsening rash or itching [see Warnings and Precautions (5.6)].Hepatotoxicity
Inform patients of the possibility of developing liver function abnormalities and serious hepatic toxicity. Advise patients to immediately contact their healthcare provider if signs of liver failure occur, including jaundice, anorexia, bleeding or bruising [see Warnings and Precautions (5.7)].Fatigue
Advise patients that BENDEKA (bendamustine hydrochloride) injection may cause tiredness and to avoid driving any vehicle or operating any dangerous tools or machinery if they experience this side effect [see Adverse Reactions (6.1)].Nausea and Vomiting
Advise patients that BENDEKA (bendamustine hydrochloride) injection may cause nausea and/or vomiting. Patients should report nausea and vomiting so that symptomatic treatment may be provided [see Adverse Reactions (6.1)].Diarrhea
Advise patients that BENDEKA (bendamustine hydrochloride) injection may cause diarrhea. Patients should report diarrhea to the physician so that symptomatic treatment may be provided [see Adverse Reactions (6.1)].Non-melanoma Skin Cancer (NMSC)
Advise patients to undergo regular skin cancer screenings, and to report any suspicious skin changes to their healthcare provider [see Warnings and Precautions (5.8)].Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.10), Use in Specific Populations (8.2, 8.3) and Nonclinical Toxicology (13.1)]. Advise female patients of reproductive potential to use effective contraception during treatment with BENDEKA and for 6 months after the last dose [see Use in Specific Populations (8.1, 8.3)]. Advise males with female partners of reproductive potential to use effective contraception during treatment with BENDEKA and for 3 months after the last dose [see Use in Specific Populations (8.3), and Nonclinical Toxicology (13.1)].Lactation
Advise females not to breastfeed during treatment with BENDEKA and for 1 week after the last dose [see Use in Specific Populations (8.2)].Infertility
Advise males of reproductive potential that BENDEKA may impair fertility [see Use in Specific Populations (8.3)].
BEN-012
Manufactured For:
Teva Pharmaceuticals
400 Interpace Parkway #3,
Parsippany, NJ 07054
All rights reserved.
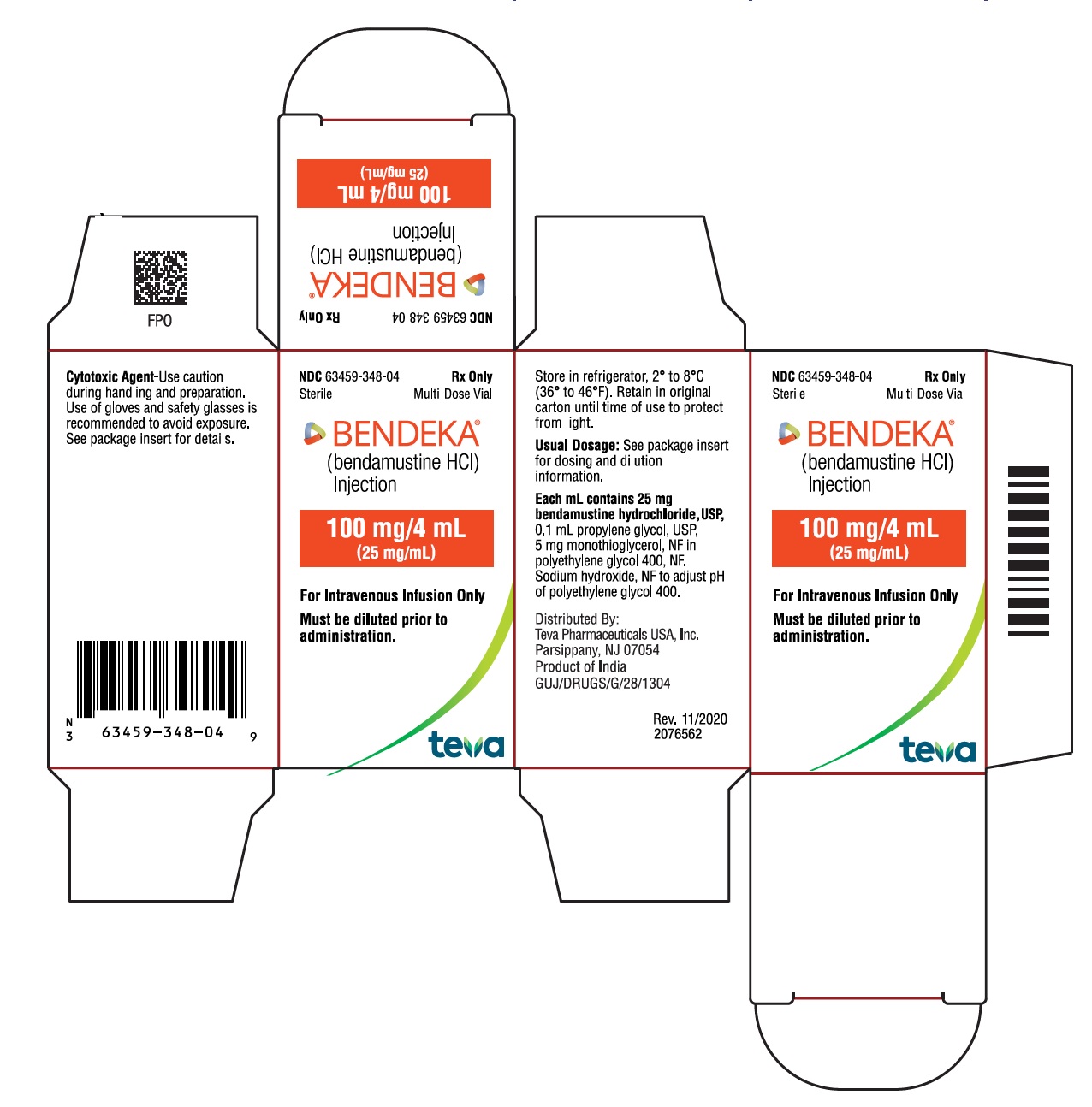
- PRINCIPAL DISPLAY PANEL - NDC: 63459-348-04 - Carton Label
-
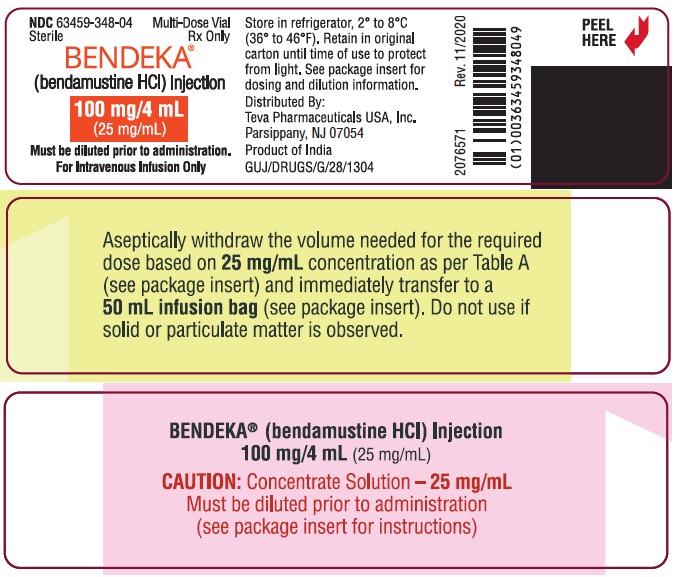
PRINCIPAL DISPLAY PANEL - NDC: 63459-348-04 - Vial Label
NDC 63459-348-04
SterileMulti-Dose Vial
Rx OnlyBENDEKA™
(bendamustine HCl) Injection100 mg/4 mL
(25 mg/mL)Must be diluted prior to administration.
For Intravenous Infusion Only
Store in refrigerator, 2° to 8°C (36° to 46 °F). Retain in original carton until time of use to protect from light. See package insert for dosing and dilution information.
Distributed By:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
Product of India
GUJ/DRUGS/G/28/1304
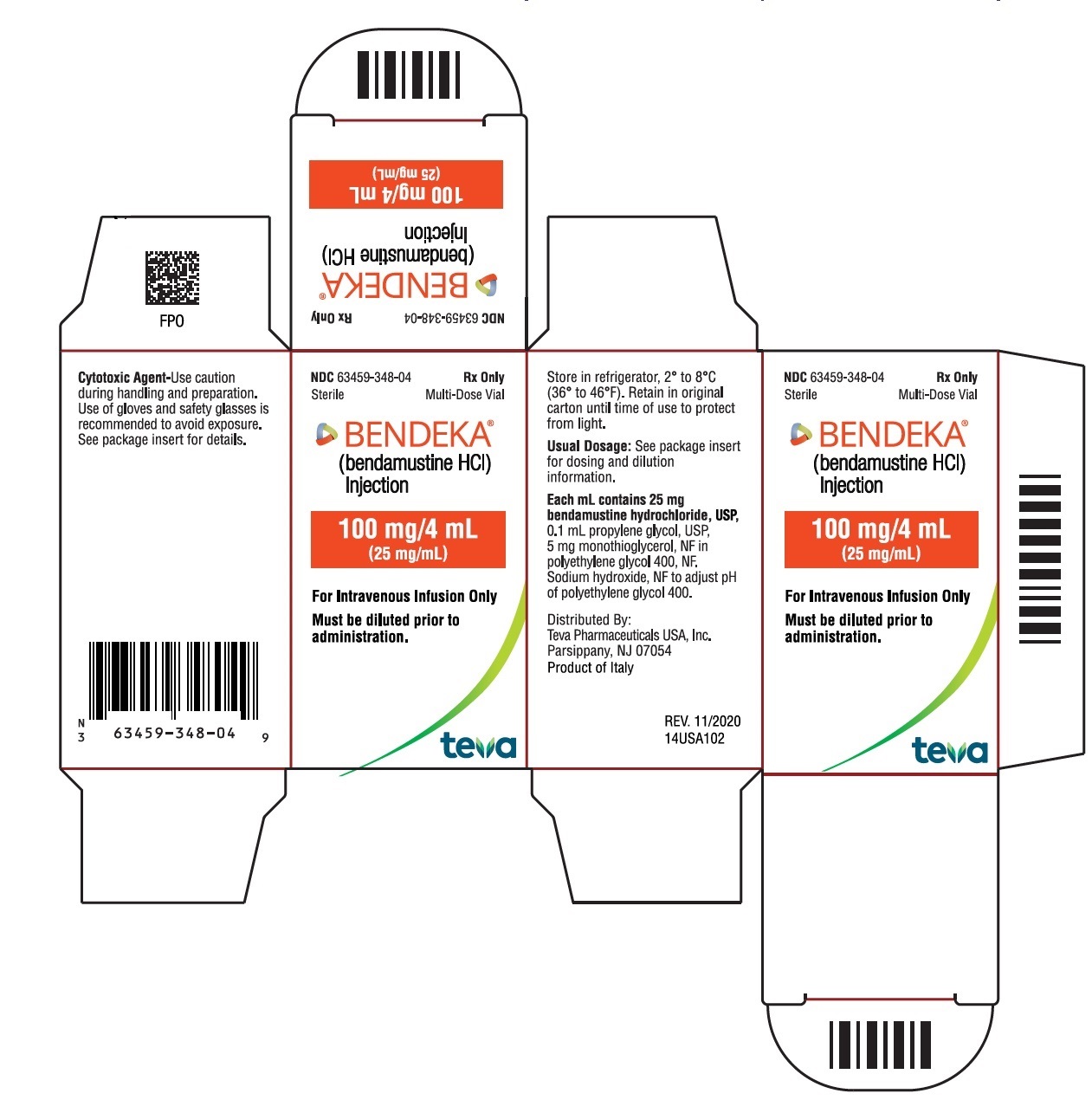
- PRINCIPAL DISPLAY PANEL - NDC: 63459-348-04 - Alternate Carton Label
-
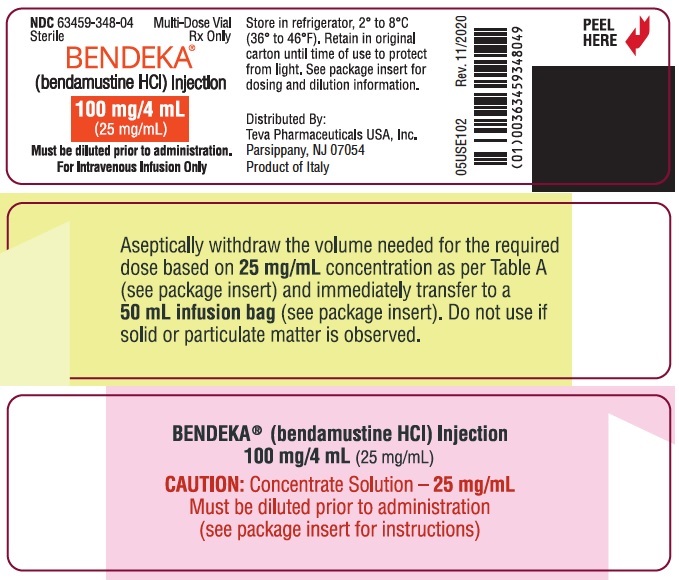
PRINCIPAL DISPLAY PANEL - NDC: 63459-348-04 - Alternate Vial Label
NDC 63459-348-04
SterileMulti-Dose Vial
Rx OnlyBENDEKA™
(bendamustine HCl) Injection100 mg/4 mL
(25 mg/mL)Must be diluted prior to administration.
For Intravenous Infusion Only
Store in refrigerator, 2° to 8°C (36° to 46 °F). Retain in original carton until time of use to protect from light. See package insert for dosing and dilution information.
Distributed By:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
Product of Italy
-
INGREDIENTS AND APPEARANCE
BENDEKA
bendamustine hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63459-348 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENDAMUSTINE HYDROCHLORIDE (UNII: 981Y8SX18M) (BENDAMUSTINE - UNII:9266D9P3PQ) BENDAMUSTINE HYDROCHLORIDE 25 mg in 1 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) MONOTHIOGLYCEROL (UNII: AAO1P0WSXJ) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63459-348-04 1 in 1 CARTON 12/08/2015 1 4 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208194 12/08/2015 Labeler - Teva Pharmaceuticals USA, Inc. (183236314)