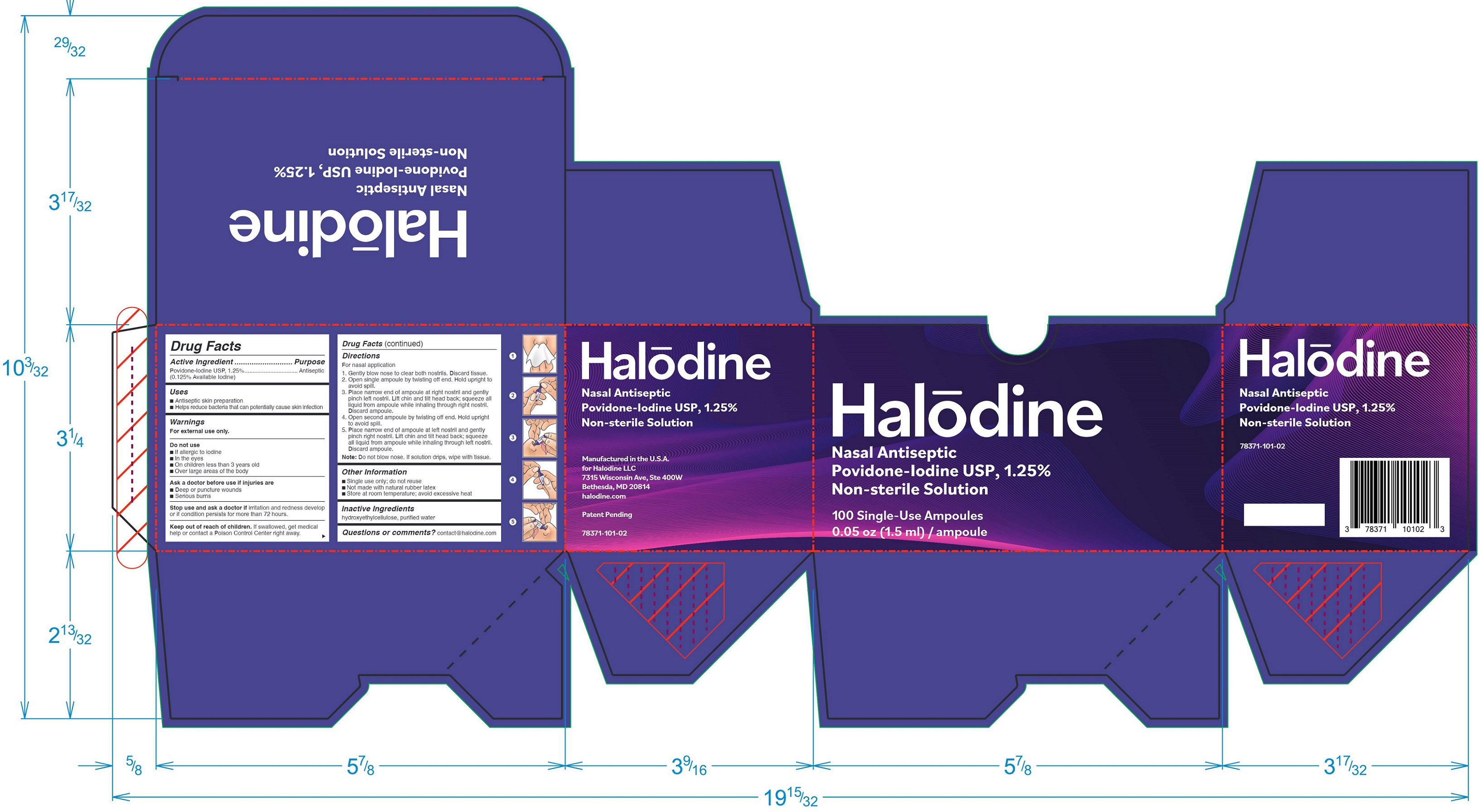
Label: HALODINE NASAL ANTISEPTIC- povidone-iodine solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 78371-101-02 - Packager: Halodine LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 14, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
- Warnings
-
Directions
For nasal application
- Gently blow nose to clear both nostrils. Discard tissue.
- Open single ampoule by tearing off the narrow end along line. To avoid spill while opening, only hold flat foil around raised body of ampoule and maintain ampoule with narrow end pointing up.
- Place narrow end of ampoule at right nostril and gently pinch left nostril. Lift chin and tilt head back; squeeze all liquid from ampoule while inhaling through right nostril. Discard ampoule.
- Open second ampoule by tearing off the narrow end along line. Avoid spill.
- Place narrow end of ampoule at left nostril and gently pinch right nostril. Lift chin and tilt head back; squeeze all liquid from ampoule while inhaling through left nostril. Discard ampoule.
Note: Do not blow nose. If solution drips, wipe with tissue.
- Other Information
- Inactive Ingredients
- Questions or comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
HALODINE NASAL ANTISEPTIC
povidone-iodine solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:78371-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 1.25 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78371-101-02 100 in 1 CARTON 06/15/2020 1 1.5 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 06/15/2020 Labeler - Halodine LLC (117526113)