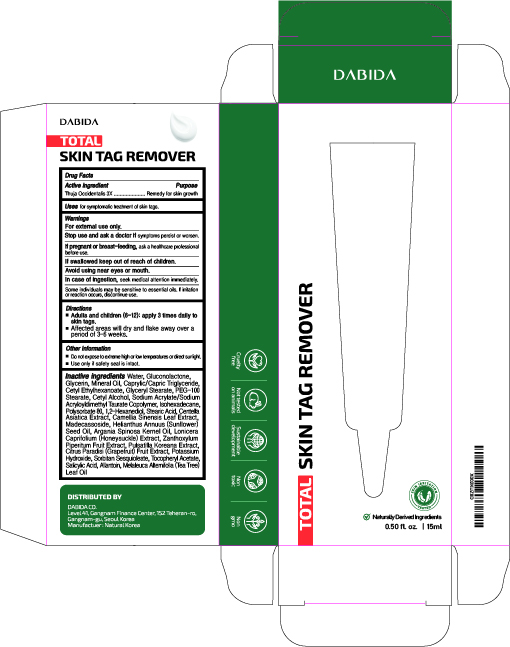
Label: TOTAL SKIN TAG REMOVER- thuja occidentalis cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 74646-0015-1 - Packager: Natural Korea Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated August 11, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TOTAL SKIN TAG REMOVER
thuja occidentalis creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74646-0015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength THUJA OCCIDENTALIS LEAF (UNII: 0T0DQN8786) (THUJA OCCIDENTALIS LEAF - UNII:0T0DQN8786) THUJA OCCIDENTALIS LEAF 3 [hp_X] in 15 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) GREEN TEA LEAF (UNII: W2ZU1RY8B0) ARGAN OIL (UNII: 4V59G5UW9X) LONICERA CAPRIFOLIUM FLOWER (UNII: 5N1WD9784U) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) GLUCONOLACTONE (UNII: WQ29KQ9POT) GLYCERIN (UNII: PDC6A3C0OX) MINERAL OIL (UNII: T5L8T28FGP) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) CETYL ALCOHOL (UNII: 936JST6JCN) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) ISOHEXADECANE (UNII: 918X1OUF1E) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) STEARIC ACID (UNII: 4ELV7Z65AP) CENTELLA ASIATICA (UNII: 7M867G6T1U) MADECASSOSIDE (UNII: CQ2F5O6YIY) SUNFLOWER OIL (UNII: 3W1JG795YI) ZANTHOXYLUM PIPERITUM FRUIT PULP (UNII: 7PFC2VA251) PULSATILLA KOREANA WHOLE (UNII: 5R35881OBK) GRAPEFRUIT (UNII: O82C39RR8C) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) SALICYLIC ACID (UNII: O414PZ4LPZ) ALLANTOIN (UNII: 344S277G0Z) TEA TREE OIL (UNII: VIF565UC2G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74646-0015-1 1 in 1 BOX 08/12/2020 1 15 mL in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/12/2020 Labeler - Natural Korea Co., Ltd. (688729438) Establishment Name Address ID/FEI Business Operations Natural Korea Co., Ltd. 688729438 manufacture(74646-0015)