Label: STRIANT- testosterone tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 55056-3060-1 - Packager: Columbia Laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: New Drug Application
Drug Label Information
Updated November 19, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Striant® (testosterone buccal system) is designed to adhere to the gum or inner cheek. It provides a controlled and sustained release of testosterone through the buccal mucosa as the buccal system gradually hydrates. Insertion of Striant® twice a day, in the morning and in the evening, provides continuous systemic delivery of testosterone.
Striant® is a white to off-white colored, monoconvex, tablet-like, mucoadhesive buccal system. Striant® adheres to the gum tissue above the incisors, with the flat surface facing the cheek mucosa.
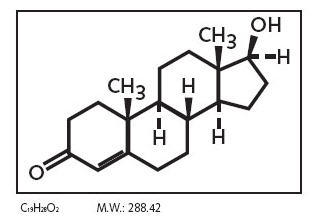
The active ingredient in Striant® is testosterone. Each buccal system contains 30 mg of testosterone. Testosterone USP is practically white crystalline powder chemically described as 17-beta hydroxyandrost-4-en-3one.
Chemical Structure:
Other pharmacologically inactive ingredients in Striant® are anhydrous lactose NF, carbomer 934P, hypromellose USP, magnesium stearate NF, lactose monohydrate NF, polycarbophil USP, colloidal silicon dioxide NF, starch NF and talc USP.
-
CLINICAL PHARMACOLOGY
Striant® delivers physiologic amounts of testosterone to the systemic circulation, thereby producing circulating testosterone concentrations in hypogonadal males that approximate physiologic levels seen in healthy young men (300 - 1050 ng/dL).
Testosterone - General Androgen Effects
Endogenous androgens, including testosterone and dihydrotestosterone (DHT) are responsible for the normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include the growth and maturation of prostate, seminal vesicles, penis, and scrotum; the development of male hair distribution, such as facial, pubic, chest, and axillary hair; laryngeal enlargement, vocal chord thickening, and alterations in body musculature and fat distribution. Testosterone and DHT are necessary for the normal development of secondary sex characteristics.
Male hypogonadism results from insufficient production of testosterone and is characterized by low serum testosterone concentrations. Symptoms associated with male hypogonadism include impotence and decreased sexual desire, fatigue and loss of energy, mood depression, regression of secondary sexual characteristics and osteoporosis. Hypogonadism is a risk factor for osteoporosis in men.
Drugs in the androgen class also promote retention of nitrogen, sodium, potassium, phosphorus, and decreased urinary excretion of calcium. Androgens have been reported to increase protein anabolism and decrease protein catabolism. Nitrogen balance is improved only when there is sufficient intake of calories and protein.
Androgens are responsible for the growth spurt of adolescence and for the eventual termination of linear growth brought about by fusion of the epiphyseal growth centers. In children, exogenous androgens accelerate linear growth rates but may cause a disproportionate advancement in bone maturation. Use by children and adolescents over long periods may result in fusion of the epiphyseal growth centers and termination of the growth process. Androgens have been reported to stimulate the production of red blood cells by enhancing the production of erythropoietin.
During exogenous administration of androgens, endogenous testosterone release may be inhibited through feedback inhibition of pituitary luteinizing hormone (LH). At large doses of exogenous androgens, spermatogenesis may also be suppressed through feedback inhibition of pituitary follicle-stimulating hormone (FSH).
Pharmacokinetics
Absorption
When applied to the buccal mucosa, Striant® slowly releases testosterone, allowing for absorption of testosterone through gum and cheek surfaces that are in contact with the buccal system. Since venous drainage from the mouth is to the superior vena cava, trans-buccal delivery of testosterone circumvents first-pass (hepatic) metabolism.
Following the initial application of Striant®, the serum testosterone concentration rises to a maximum within 10-12 hours. The mean maximum (C max ) and mean average serum total testosterone concentrations for the 12 hour dosing period (C avg(0-12) ) are within the normal physiologic range.
Striant® is intended for twice daily dosing. Serum concentrations of testosterone reach steady-state levels after the second dose of twice daily Striant® dosing. Following removal of Striant®, the serum testosterone concentration decreases to a level below the normal range within 2-4 hours.
With twice-daily repeated dosing, mean pharmacokinetic parameters at steady-state for total testosterone serum concentration were very similar between studies of 7-day and 12-week dosing durations. Mean C avg(0-24) across the studies ranged from 520 to 550 ng/dL and these mean values were within the physiologic range (see Table 1).
Table 1. Mean (±SD) Steady-State Serum Total Testosterone Concentrations During Treatment with Striant® (on Final Day of Treatment) Study 1 Study 2 12-weeks
(N=82)7-days
(N=29)C avg(0-24) (ng/dL) 520 (±205) 550 (±169) C max(0-24) (ng/dL) 970 (±442) 910 (±319) C min(0-24) (ng/dL) 290 (±130) 320 (±131) Although no specific food effect study was conducted, pivotal Phase 3 study results showed that consumption of food and beverage did not significantly affect the absorption of testosterone from Striant®.
The effects of toothbrushing, mouthwashing, chewing gum and alcoholic beverages on the use and absorption of Striant® were not investigated in controlled studies, however, Phase 3 clinical studies permitted patients to do these activities indicating the use of Striant® was not significantly affected by these activities.
Distribution
Circulating testosterone is chiefly bound in the serum to sex hormone-binding globulin (SHBG) and albumin. The albumin-bound fraction of testosterone easily dissociates from albumin and is presumed to be bioactive. The portion of testosterone bound to SHBG is not considered biologically active. The amount of SHBG in the serum and the total testosterone level will determine the distribution of bioactive and nonbioactive androgen. SHBG-binding capacity is high in prepubertal children, declines during puberty and adulthood, and increases again during the later decades of life. Approximately 40% of testosterone in plasma is bound to SHBG, 2% remains unbound (free) and the rest is bound to albumin and other proteins.
Metabolism
There is considerable variation in the half-life of testosterone as reported in the literature, ranging from ten to 100 minutes. Testosterone is metabolized to various 17-keto steroids through two different pathways, and the major active metabolites are estradiol and dihydrotestosterone (DHT). DHT binds with greater affinity to SHBG than does testosterone. In many tissues the activity of testosterone appears to depend on reduction to DHT, which binds to cytosol receptor proteins. The steroid-receptor complex is transported to the nucleus where it initiates transcription and cellular changes related to androgen action. In reproductive tissues, DHT is further metabolized to 3-alpha and 3-beta androstanediol.
Mean DHT concentrations increase in parallel with testosterone concentrations during Striant® treatment. After 24 hours of treatment, mean DHT serum concentrations are within normal range. The mean steady-state T/DHT ratio during treatment with Striant® remained within normal limits as determined by the analytical laboratory involved with the clinical trials. These ratios ranged from approximately 9-12.
Excretion
About 90% of a dose of testosterone given intramuscularly is excreted in the urine as glucuronic and sulfuric acid conjugates of testosterone and its metabolites; about 6% of a dose is excreted in the feces, mostly in the unconjugated form. Inactivation of testosterone occurs primarily in the liver.
Clinical Studies
Striant® was evaluated in a multicenter, open-label, single arm, Phase 3 trial in 98 hypogonadal men (Study 1). In this study, Striant® was administered twice daily for 12 weeks. The mean age was 53.6 years (range 20 to 75 years). Overall, 68 (69.4%) patients were Caucasian, 9 (9.2%) were African-American, 15 (15.3%) were Hispanic, 4 (4.1%) were Asian, and 2 (2.0%) were of another ethnic origin. At baseline, ten patients (10.2%) reported current use of tobacco and forty-one (41.8%) drank alcohol. Of 82 patients who completed the trial and had sufficient data for full analysis, 86.6% had mean serum testosterone concentration (C avg(0-24) ) values within the physiologic range.
The mean (±SD) time-averaged steady-state daily testosterone concentration (C avg(0-24) ) at Week 12 was 520 (±205) ng/dL compared with a mean of 149 (±99) ng/dL at Baseline. At Week 12, the mean percentage of time over the 24-hour sampling period that total testosterone concentrations remained within the normal range of 300 - 1050 ng/dL was 76%. Table 1 above provides the steady-state serum testosterone concentrations in greater detail.
Striant® was also evaluated in a 7-day multicenter, open-label, parallel study comparing Striant® and an approved testosterone transdermal system (Study 2). In this study, Striant® was again administered twice daily. On Day 7, the mean C avg(0-24) for the 29 patients who received Striant® was 550 (±169) ng/dL compared with a mean of 119 (±78) ng/dL at Baseline. At Day 7, the mean percentage of time for Striant® over the 24-hour sampling period that testosterone concentrations remained within the physiologic range of 300-1050 ng/dL was 84%. Additional pharmacokinetic data for this study are presented in Table 1 above.
Figure 1 below shows the mean total testosterone serum concentration versus time at steady-state for two representative consecutive dosing intervals from both the 7-day and 12 week studies. The figure shows that the concentration-time curves for the different duration studies are consistent.
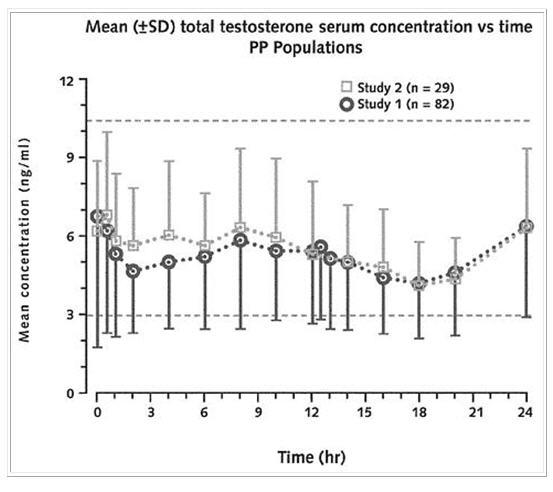
Figure 1: Mean (SD) total testosterone concentration-time curves for two consecutive dosing intervals at steady-state for both the 12- week study (Study 1) and the 7-day study (Study 2) of Striant®. (The horizontal dotted lines represent the upper and lower limit of normal for the normal physiologic range in healthy adult males).
In both clinical trials, mean DHT concentrations increased in parallel with testosterone concentrations, with the total testosterone/DHT ratio (9 - 12) indicating no alteration in metabolism of testosterone to DHT in testosterone deficient men treated with Striant® as compared with young, healthy eugonadal men.
During continuous treatment there was no accumulation of testosterone, and mean total testosterone, free testosterone, and DHT were maintained within their physiologic ranges.
-
INDICATIONS AND USAGE
Striant® is indicated for replacement therapy in males for conditions associated with a deficiency or absence of endogenous testosterone:
Primary hypogonadism (congenital or acquired) - testicular failure due to cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome, orchidectomy, Klinefelter's syndrome, chemotherapy, or toxic damage from alcohol or heavy metals. These men usually have low serum testosterone levels and gonadotropins (FSH, LH) above the normal range.
Hypogonadotropic hypogonadism (congenital or acquired) -- idiopathic gonadotropin or LHRH deficiency, or pituitary hypothalamic injury from tumors, trauma, or radiation. These patients have low serum testosterone levels but have gonadotropins in the normal or low range.
-
CONTRAINDICATIONS
Androgens are contraindicated in men with carcinoma of the breast or known or suspected carcinoma of the prostate.
Striant® is not indicated for use in women, and must not be used in women. Testosterone supplements may cause fetal harm.
Striant® should not be used in patients with known hypersensitivity to any of its ingredients, including testosterone USP that is chemically synthesized from soy.
-
WARNINGS
- Prolonged use of high doses of orally active 17-alpha-alkyl androgens (e.g., methyltestosterone) have been associated with serious hepatic adverse effects (peliosis hepatis, hepatic neoplasms, cholestatic hepatitis, and jaundice). Peliosis hepatis can be a life-threatening or fatal complication. Long-term therapy with testosterone enanthate, which elevates blood levels for prolonged periods, has produced multiple hepatic adenomas. Testosterone is not known to produce these adverse effects.
- Geriatric patients treated with androgens may be at an increased risk for the development of prostatic hyperplasia and prostatic carcinoma.
- Geriatric patients and other patients with clinical or demographic characteristics that are recognized to be associated with an increased risk of prostate cancer should be evaluated for the presence of prostate cancer prior to initiation of testosterone replacement therapy. In men receiving testosterone replacement therapy, surveillance for prostate cancer should be consistent with current practices for eugonadal men (see PRECAUTIONS: Carcinogenesis, Mutagenesis, Impairment of Fertility and Laboratory Tests).
- Edema with or without congestive heart failure may be a serious complication in patients with preexisting cardiac, renal, or hepatic disease. In addition to discontinuation of the drug, diuretic therapy may be required.
- Gynecomastia frequently develops and occasionally persists in patients being treated for hypogonadism.
- The treatment of hypogonadal men with testosterone esters may potentiate sleep apnea in some patients especially those with risk factors such as obesity or chronic lung diseases.
-
PRECAUTIONS
Striant® is applied to the upper gum just above the incisor tooth on either side of the mouth. Long-term data on gum safety is available for 117 patients and 51 patients with at least 6 months and 1 year of exposure, respectively. While the available data supports the overall oral safety of Striant®, longer-term data is not currently available and studies continue. Until such longer-term data become available, it is recommended that patients regularly inspect their own gum region where Striant® is applied. Any abnormal finding should be brought promptly to the attention of the patient's physician. In such circumstances, dental consultation may be appropriate.
General
The physician should instruct patients to report any of the following:
- Too frequent or persistent erections of the penis.
- Any nausea, vomiting, changes in skin color, or ankle swelling.
- Breathing disturbances, including those associated with sleep.
Information for Patients
Advise patients to carefully read the attached patient leaflet accompanying each carton of Striant® blister packaged tablets.
Advise patients to regularly inspect the gum region where they apply Striant® and to report any abnormality to their health care professional.
Laboratory Tests
- Hemoglobin and hematocrit levels should be checked periodically (to detect polycythemia) in patients on long-term androgen therapy.
- Liver function, prostate specific antigen (PSA), cholesterol and high-density lipoprotein should be checked periodically.
- Serum total testosterone concentrations may be checked four to twelve weeks after initiating treatment with Striant®. To capture the maximum serum concentration, an early morning sample (just prior to applying the A.M. dose) is recommended. In the infrequent circumstance where the total testosterone concentration in this sample is excessive, therapy with Striant® should be discontinued and an alternative treatment considered.
Drug interactions
Oxyphenbutazone
Concurrent administration of oxyphenbutazone and androgens may result in elevated serum levels of oxyphenbutazone.
Drug/Laboratory Test Interactions
Androgens may decrease levels of thyroxin-binding globulin, resulting in decreased total T4 serum levels and increased resin uptake of T3 and T4. Free thyroid hormone levels remain unchanged, however, and there is no clinical evidence of thyroid dysfunction.
Carcinogenesis, mutagenesis, impairment of fertility
Animal data
Testosterone has been tested by subcutaneous injection and implantation in mice and rats. In mice, the implant induced cervical-uterine tumors, which metastasized in some cases. There is suggestive evidence that injection of testosterone into some strains of female mice increases their susceptibility to hepatoma. Testosterone is also known to increase the number of tumors and decrease the degree of differentiation of chemically induced carcinomas of the liver in rats.
Human data
There were rare reports of hepatocellular carcinoma in patients receiving long-term therapy with androgens in high doses. Withdrawal of the drugs did not lead to regression of the tumors in all cases.
Striant® has been evaluated in patients for 1 year without reports of cancer related to the product. However, safety in patients beyond 1 year has not been established.
Geriatric patients treated with androgens may be at an increased risk for the development of prostatic hyperplasia and prostatic carcinoma.
Geriatric patients and other patients with clinical or demographic characteristics that are recognized to be associated with an increased risk of prostate cancer should be evaluated for the presence of prostate cancer prior to initiation of testosterone replacement therapy.
In men receiving testosterone replacement therapy, surveillance for prostate cancer should be consistent with current practices for eugonadal men.
Pediatric Use
Safety and effectiveness in pediatric male patients below the age of 18 have not yet been established
Geriatric Use
Of the total number of subjects in clinical studies of Striant®, 51 patients (16.5 percent) were 65 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. However, in Study 1, in patients 65 years of age and older, the total testosterone C avg(0-24) value was higher by 12.7% compared to patients less than 65 years of age. In addition, the total T to DHT area-under-the curve ratio was lower in the older population compared to the younger population by 15.6%. These differences may not be clinically significant.
-
ADVERSE REACTIONS
In all clinical studies combined, a total of 308 patients were treated with Striant® for up to 12 months
Twelve Week Trials
In the pivotal, Phase 3, open-label controlled study (Study 1), 98 patients received Striant® for up to 12 weeks. Adverse events judged possibly, probably, or definitely related to the use of Striant® and reported by >/= 1% of patients in Study 1 are listed in Table 2.
Table 2. Incidences of Adverse Events Possibly, Probably or Definitely Related Use of Striant® in Study 1 Adverse event Striant®
(n=98)
Gum or Mouth Irritation 9.2% Taste Bitter 4.1% Gum Pain 3.1% Gum Tenderness 3.1% Headache 3.1% Gum Edema 2.0% Taste Perversion 2.0% Please see "Gum-related adverse events and gum examinations" subsection for further information. The majority of gum-related adverse events were transient. Gum irritation generally resolved in 1 to 8 days. Gum tenderness resolved in 1 to 14 days.
The following adverse events judged possibly, probably or definitely related to the use of Striant® occurred in 1 patient each in Study 1: abdominal cramp, acne, anxiety, asthma (acute), breast enlargement, breast pain, buccal mucosal roughening, difficulty in micturition, fatigue, gingivitis, gum blister, gustatory sense diminished, hematocrit increased, lipids serum increased, liver function tests abnormal, nose edema, stinging of lips, and toothache.
There was one additional 12-week study in 12 patients. In this study, additional adverse events judged at least possibly related to Striant® and reported by 1 patient each included emotional lability and hypertension.
Long-Term Extension Trials
In two long-term extension trials, a total of 117 and 51 patients received Striant® for at least 6 months and 1 year, respectively.
Of 117 patients treated for at least 6 months, adverse events judged possibly, probably, or definitely related to treatment and reported by 1 patient each included: anxiety, buccal inflammation, depression, dry mouth, gastrointestinal disorder, gum redness, hypertension, infection, medication error, nausea, pruritis, renal function abnormal, stomatitis, taste bitter, taste perversion, and toothache. Polycythemia and increased serum prostate specific antigen (PSA) were reported in three and two patients, respectively.
Adverse events reported in the 51 patients treated for at least one year were similar to those reported after 6 months of treatment and lower in incidence.
Gum-related adverse events and gum examinations
In the pivotal controlled study (Study 1), all reported gum-related adverse events were collected and gum examinations were conducted at Baseline and every month thereafter.
In Study 1, a total of 16 patients reported 19 gum-related adverse events. Of these, ten patients (10.2%) reported 12 events of mild intensity, four patients (4.1%) reported 5 events of moderate intensity, and two patients (2.0%) reported 2 events of severe intensity. Most of these events were judged probably or definitely related to treatment with Striant®. Four patients (4.1%) discontinued treatment with Striant® due to gum or mouth-related adverse events including two with severe gum irritation, one with mouth irritation, and one with "bad taste in mouth". The majority of gum-related adverse events were transient. Gum irritation generally resolved in 1 to 8 days. Gum tenderness resolved in 1 to 14 days.
In Study 1, monthly gum examinations were conducted to assess for gingivitis, gum edema, oral lesions, ulcerations or leukoplakia. No cases of ulceration or leukoplakia were observed. No new oral lesions were observed. Gingivitis was common at Baseline (32.6%), and was reduced at Week 4 (10.2%), Week 8 (10.2%) and Week 12 (11.2%). Similar findings were seen for gum edema.
In the two long-term extension trials, gum examinations were conducted every 3 months while on treatment. In one of these trials, no patient had a gum abnormality, and in the other trial, moderate gingivitis and mild gum edema were reported by 1 patient each.
- DRUG ABUSE AND DEPENDENCE
-
OVERDOSAGE
There is one report of acute overdosage with testosterone enanthate injection: testosterone levels of up to 11,400 ng/dL were implicated in a cerebrovascular accident.
Oral ingestion of Striant® is not expected to result in clinically significant serum testosterone concentrations due to extensive first-pass (hepatic) metabolism.
-
DOSAGE AND ADMINISTRATION
The recommended dosing schedule for Striant® is the application of one buccal system (30 mg) to the gum region twice daily; morning and evening (about 12 hours apart). Striant® should be placed in a comfortable position just above the incisor tooth (on either side of the mouth). With each application, Striant® should be rotated to alternate sides of the mouth.
Upon opening the packet, the rounded side surface of the buccal system should be placed against the gum and held firmly in place with a finger over the lip and against the product for 30 seconds to ensure adhesion. Striant® is designed to stay in position until removed. If the buccal system fails to properly adhere to the gum or should fall off during the 12-hour dosing interval, the old buccal system should be removed and a new one applied. If the buccal system falls out of position within 4 hours prior to the next dose, a new buccal system should be applied and it may remain in place until the time of next regularly scheduled dosing.
Patients should take care to avoid dislodging the buccal system. Patients should check to see if Striant® is in place following toothbrushing, use of mouthwash and consumption of food or alcoholic/non-alcoholic beverages. Striant® should not be chewed or swallowed. To remove Striant®, gently slide it downwards from the gum towards the tooth to avoid scratching the gum.
-
HOW SUPPLIED
Striant® (testosterone buccal system) is for buccal administration only. It contains testosterone, a Schedule III controlled substance as defined by the Anabolic Steroids Control Act.
Striant® is supplied in transparent blister packs containing 10 doses. It is white to off- white colored with a flat edge on one side and a convex surface on the other.
Striant® is debossed on its flat side, as shown below:

Each Striant® buccal system contains 30 mg of testosterone and is supplied as follows:
NDC Number Strength Package Size 55056-3060-1 30 mg 6 blister packs, 10 buccal systems per blister; 30 mg per buccal system Storage and Disposal
Store at 20-25 °C (68-77 °F) [see USP Controlled Room temperature]. Protect from heat and moisture. Damaged blister packages should not be used. Discarded Striant® buccal systems should be disposed of in household trash in a manner that prevents accidental application or ingestion by children or pets.
- SPL UNCLASSIFIED SECTION
-
Patient Information
STRIANT® CIII
(testosterone buccal system) mucoadhesiveRead the Patient Information that comes with Striant® [STRI' ant] before you start using it and each time you get a refill. There may be new information. This information does not take the place of information from your healthcare provider about your medical condition or your treatment.
What is Striant®?
Striant® is a hormone medicine that contains testosterone. It is used to treat adult men when their bodies do not make any testosterone or not enough testosterone (hypogonadism). Striant® is a white to off-white tablet-like buccal system that is applied to the upper gum area of the mouth. Striant® is not to be chewed or swallowed.
Striant® is a controlled substance (CIII) because it contains testosterone. Therefore, you should keep your Striant® in a secure place. Do not share or sell your Striant®.
Who should not use Striant®?
Do not use Striant® if you:
- have breast cancer (rare in men).
- have prostate cancer.
- are a woman (especially if you are pregnant or breast-feeding). Striant may harm the babies of pregnant and breast-feeding women.
- are allergic to Striant®. The active ingredient in Striant® is testosterone USP. See the end of this leaflet for a list of all ingredients in Striant®.
Tell your doctor if you have or had:
- problems urinating due to an enlarged prostate.
- liver problems.
- kidney problems.
- heart problems.
- lung problems.
- diabetes.
- weight problems (obesity).
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Some medicines may cause serious side effects if taken while you also take Striant®. Some medicines may affect how Striant® works, or Striant® may affect how your other medicines work. Be sure to tell your doctor if you use insulin for diabetes. Your dose of insulin may need to be adjusted if you use Striant®.
How should I use Striant®?
Use Striant® twice a day, once in the morning and once at night (about 12 hours apart). You may find it convenient to apply morning dose after brushing your teeth following breakfast and evening dose following your evening meal.
Striant® should be applied as follows:
- Tear off individual unit then start at the corner tab and peel off paper backing. Push buccal system through foil from the front. You will notice that Striant® is curved on one side and flat on the other side. The flat side has a marking on it (company logo).

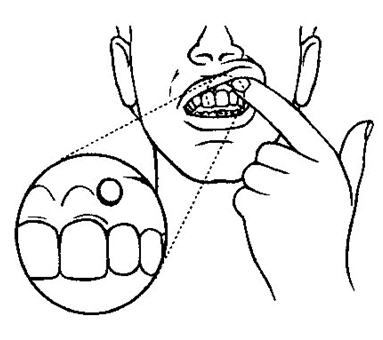
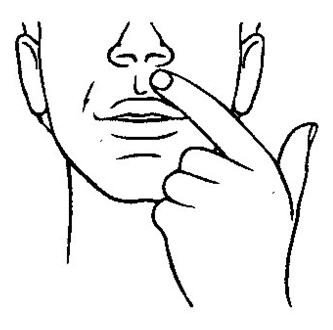
- Before you apply Striant®, locate the area on your upper gum, just above either the left or right incisor (see picture). The incisor is the tooth just to the right or left of your two front teeth.
- Place the flat side of the Striant® system on your fingertip. Gently push the curved side of Striant® against your upper gum in the area shown. Push the Striant® system up as high as it will go on the gum. If you have applied Striant® correctly, the flat side will be facing your cheek.
- Using your finger on the outside of your upper lip, hold the Striant® buccal system in place for 30 seconds (see picture). This will make the buccal system stick to your gum or cheek.
- As the Striant® buccal system absorbs moisture from your mouth, it will begin to soften and will mold to the shape of your gum. You should be aware that Striant® does not dissolve completely, but will remain in place for 12 hours. Striant® is made to stay in place until you remove it.
- Remove Striant® by gently sliding it to the front or back of your mouth to loosen it. Then slide it downwards from your gum to your tooth. This will avoid scratching the gum.
- With each application, you should rotate Striant® to alternate sides of your mouth.
- If Striant® does not stick or falls off within the first 8 hours , remove the original system and apply a new one. This counts as replacing the first dose. Apply the next system about 12 hours after the original buccal system was applied.
- If Striant® falls off after 8 hours but before 12 hours , replace the original buccal system. This replacement can serve as the second dose for that day.
- If Striant® sticks to your cheek and not your gum, this is acceptable. Do not replace the buccal system if this should happen.
- Check to see if Striant® is in place following toothbrushing, use of mouthwash and consumption of food or alcoholic/nonalcoholic beverages. If Striant® does not stick or falls off, follow the above mentioned directions to replace with a new system.
What are the possible side effects of testosterone replacement therapy?
Inform your doctor immediately if any of the following symptoms appear while using Striant®.
-
Liver problems. Tell your doctor if:
- Your skin or white part of your eyes turns yellow (jaundice).
- Your urine turns dark.
- Your bowel movements (stool) turns light in color.
- You don't feel like eating for several days or longer.
- You feel sick to your stomach (nausea).
- You have lower abdominal pain (stomach).
- Problems urinating. Tell your doctor if you develop problems urinating while using Striant®. Older patients who use testosterone replacement therapies may have an increased chance of developing prostate enlargement or prostate cancer.
- Extra fluid in the body (edema). Edema can be dangerous if you have heart, kidney or liver problems. Tell your doctor if your ankles and legs swell or if you put on weight quickly.
- Breathing problems, including a sleep problem called "sleep apnea". Sleep apnea is when you stop breathing for short times while you are sleeping. This happens more in patients who are overweight or who have lung disease. Tell your doctor if you have breathing problems or if you or your partner notice changes in your breathing when you are sleeping.
- Penile erections that are painful, that occur too frequently, or that last for too long a duration.
- Breast enlargement - which sometimes does not go away.
- Emotional changes - such as depression.
Your doctor may do blood tests to check your red blood cells, liver function, cholesterol levels, testosterone levels and prostate (PSA) while you are using Striant®, to see how Striant® is affecting your body.
Striant® may also cause these side effects:
- redness, irritation, swelling and pain at the gum application site.
- gum infection (gingivitis)-gum side effects are usually temporary, and should resolve within several days. However, some gum side effects may last up to two weeks. If you should have gum side effects, they usually resolve while taking Striant®. Any abnormal finding should be brought to the attention of your physician.
- a change in how food tastes to you, a bitter taste in your mouth, or an unusual taste in your mouth.
- headache.
These are not all the possible side effects of Striant®. For more information, ask your doctor or pharmacist.
You should regularly examine your gums where Striant® is applied. Any abnormal finding should be brought to the attention of your physician.
How should Striant® be stored?
Keep Striant® at a temperature between 68° and 77° F (20-25° C). Protect from heat and moisture. Do not use a damaged blister package. Keep Striant® and all medicines out of the reach of children. Discarded Striant® buccal systems should be thrown away in a household trash can in a way that prevents children or pets from accidentally using or taking them.
General information about the safe and effective use of Striant®. Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use Striant® for a condition for which it was not prescribed. Do not give Striant® to other people, even if they have the same symptoms you have. It may harm them, and you should be aware that Striant® is a controlled substance.
This leaflet summarizes the most important information about Striant®. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Striant® that is written for health professionals.
What are the ingredients of Striant®?
Active Ingredient: Testosterone USP (30 mg in each buccal system)
Inactive Ingredients: anhydrous lactose NF, carbomer 934P, hypromellose USP, magnesium stearate NF, lactose monohydrate NF, polycarbophil USP, colloidal silicon dioxide NF, starch NF, and talc USP.
How is Striant® supplied?
Striant® is supplied in a transparent blister in a white card. Each card contains 10 buccal systems. There are a total of 6 blister cards (60 buccal systems) in each carton.
Rx Only
Manufactured by: Mipharm S.p.A, Milan, Italy
Manufactured for: Columbia Laboratories, Inc., Livingston, NJ 07039
US Patent Numbers: 6,248,358
others pending
© 2003 Columbia Laboratories, Inc.
PATSTN002/41005010002
USA/930874/0 - PRINCIPAL DISPLAY PANEL - 60 Buccal System Carton
-
INGREDIENTS AND APPEARANCE
STRIANT
testosterone tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55056-3060 Route of Administration BUCCAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength testosterone (UNII: 3XMK78S47O) (testosterone - UNII:3XMK78S47O) testosterone 30 mg Inactive Ingredients Ingredient Name Strength anhydrous lactose (UNII: 3SY5LH9PMK) carbomer 934 (UNII: Z135WT9208) hypromellose (UNII: 3NXW29V3WO) magnesium stearate (UNII: 70097M6I30) lactose monohydrate (UNII: EWQ57Q8I5X) polycarbophil (UNII: W25LM17A4W) silicon dioxide (UNII: ETJ7Z6XBU4) starch, corn (UNII: O8232NY3SJ) talc (UNII: 7SEV7J4R1U) Product Characteristics Color WHITE Score no score Shape ROUND Size 9mm Flavor Imprint Code Columbia;C Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55056-3060-1 6 in 1 CARTON 1 10 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021543 06/19/2003 Labeler - Columbia Laboratories, Inc. (177253754) Establishment Name Address ID/FEI Business Operations Columbia Laboratories Inc 177253754 REPACK