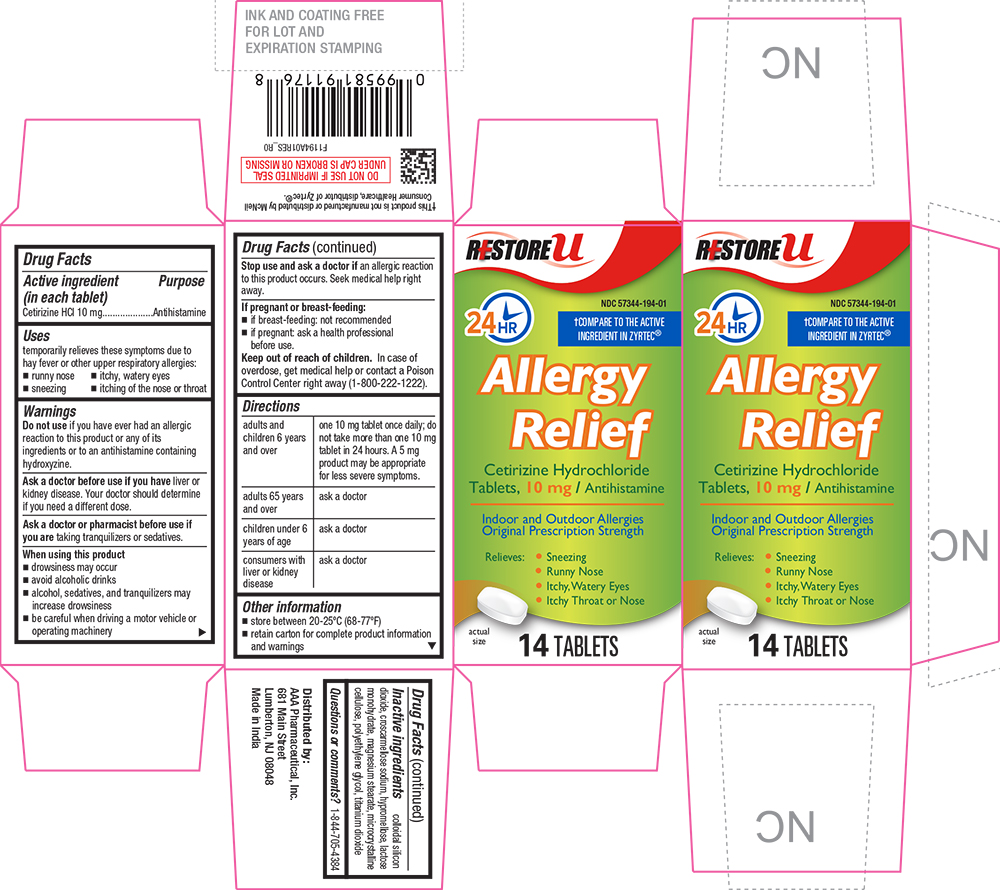
Label: ALLERGY RELIEF- cetirizine hydrochloride tablet, coated
- NDC Code(s): 57344-194-01, 57344-194-14
- Packager: AAA PHARMACEUTICAL, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 21, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each tablet)
- Purpose
- Uses
-
WARNINGS
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
-
Directions
adults and children 6 years and over one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms. adults 65 years and over ask a doctor children under 6 years of age ask a doctor consumers with liver or kidney disease ask a doctor
- Other information
- Inactive ingredients
- Questions or Comments?
-
PRINCIPAL DISPLAY PANEL
RESTORE U
24 HR
NDC 57344-194-01
COMPARE TO THE ACTIVE INGREDIENT IN ZYRTEC®
Allergy Relief
Cetirizine Hydrochloride Tablets, 10 mg / Antihistamine
Indoor and Outdoor Allergies
Original Prescription Strength
Relieves: Sneezing, Runny Nose, Itchy, Watery Eyes, Itchy Nose or Throat
actual size
14 TABLETS
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
cetirizine hydrochloride tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57344-194 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color white (white to off white) Score 2 pieces Shape RECTANGLE (rounded off rectangualr) Size 9mm Flavor Imprint Code G;4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57344-194-01 1 in 1 CARTON 10/01/2018 1 14 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:57344-194-14 1 in 1 CARTON 04/29/2022 2 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209274 10/01/2018 Labeler - AAA PHARMACEUTICAL, INC. (181192162)