Label: NIGHT TIME SLEEP-AID- diphenhydramine hcl liquid
- NDC Code(s): 53943-522-25, 53943-522-28
- Packager: Discount Drug Mart
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
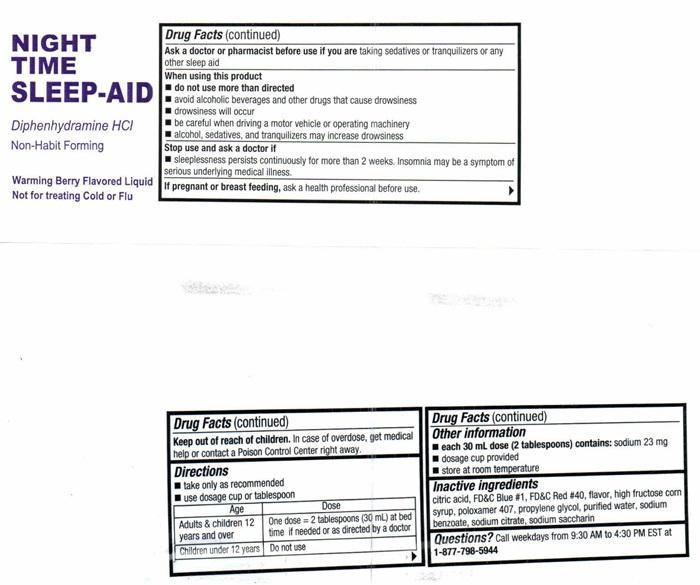
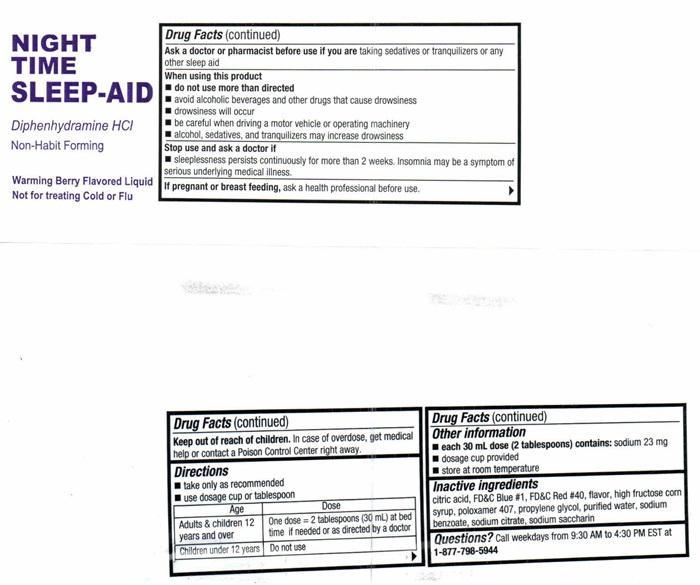
- Drug FactsActive ingredients (in each 30 mL dose cup or 2 tablespoons)
- Purpose
- Kep out of reach of children
- Uses
- Warnings
- Do not use
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast feeding,
- Directions
- Other information
- Inactive ingredients
- Questions?
-
DISCOUNT drug mart FOOD FAIR NIGHT TIME SLEEP-AID product label
* COMPARE TO THE ACTIVE INGREDIENT IN ZzzQuil™NIGHTTIME SLEEP-AID
DISCOUNT
drug mart
FOOD FAIR
NIGHT TIME SLEEP-AID
Diphenhydramine HCL
Non-Habit Forming
Warming Berry Flavored Liquid
Not for treating Cold or Flu
12 FL OZ (354 mL)
* This product is not manufactured or distributed by Proctor and Gamble owner of the registered trademark ZzzQuil™
LR-055
SATISFACTION GUARANTEED
IF DISSATISFIED, RETURN UNUSED PORTION AND PACKAGE TO THE STORE WHERE PURCHASED. IF UNABLE TO RETURN TO THE STORE, SEND REASON FOR DISSATISFACTION , NAME, ADDRESS AND EMPTY PACKAGE TO: DISCOUNT DRUG MART, 211 COMMERCE DRIVE MEDINNA, OHIO 44256

res


-
INGREDIENTS AND APPEARANCE
NIGHT TIME SLEEP-AID
diphenhydramine hcl liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53943-522 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 50 mg in 30 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) POLOXAMER 407 (UNII: TUF2IVW3M2) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE (UNII: 1Q73Q2JULR) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Product Characteristics Color Score Shape Size Flavor BERRY (BERRY) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53943-522-25 117 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/10/2013 2 NDC:53943-522-28 354 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/10/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M010 12/10/2013 Labeler - Discount Drug Mart (047741335)