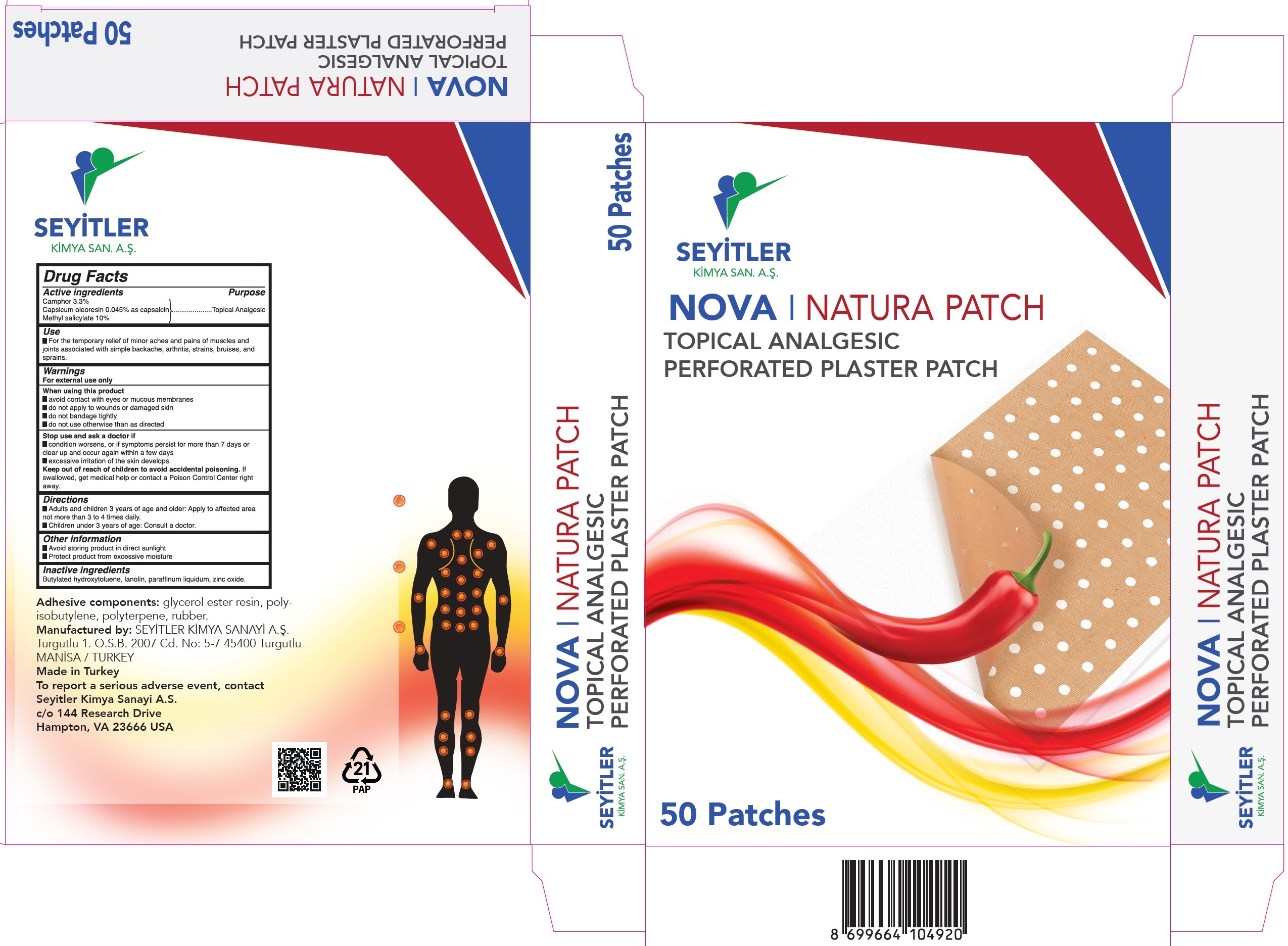
Label: NOVA NATURA TOPICAL ANALGESIC- camphor, capsicum oleoresin, methyl salicylate patch
- NDC Code(s): 84046-001-01
- Packager: SEYITLER KIMYA SANAYI ANONIM SIRKETI
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Use
-
Warnings
For external use only
When using this product
- avoid contact with eyes or mucous membranes
- do not apply to wounds or damaged skin
- do not bandage tightly
- do not use otherwise than as directed
- Directions
- Other information
- Inactive ingredients
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
NOVA NATURA TOPICAL ANALGESIC
camphor, capsicum oleoresin, methyl salicylate patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84046-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 72.17 mg in 1 g CAPSICUM OLEORESIN (UNII: UW86K581WY) (CAPSICUM OLEORESIN - UNII:UW86K581WY) CAPSICUM OLEORESIN 1.01 mg in 1 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 218 mg in 1 g Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) LANOLIN (UNII: 7EV65EAW6H) MINERAL OIL (UNII: T5L8T28FGP) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84046-001-01 1 in 1 BOX 01/02/2024 1 50 in 1 PACKET 1 2.187 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/02/2024 Labeler - SEYITLER KIMYA SANAYI ANONIM SIRKETI (631427044) Establishment Name Address ID/FEI Business Operations SEYITLER KIMYA SANAYI ANONIM SIRKETI 631427044 manufacture(84046-001)