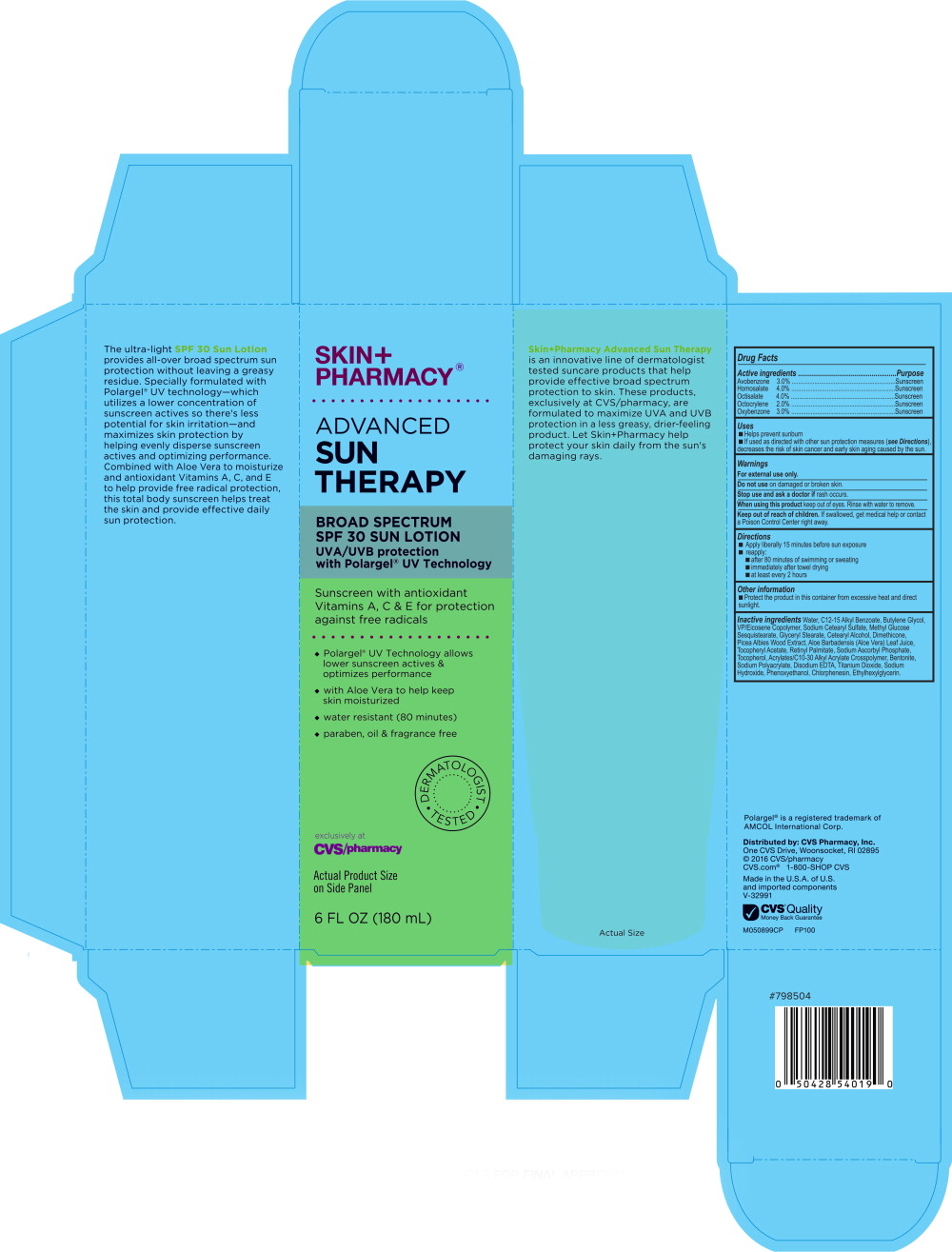
Label: SKIN PHARMACY ADVANCED SUN THERAPY BROAD SPECTRUM SPF 30 SUN- homosalate, octisalate, avobenzone, oxybenzone, octocrylene liquid
- NDC Code(s): 69842-078-01
- Packager: CVS Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 24, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
-
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging cause by the sun.
- Warnings
- Directions
- Other information
-
Inactive ingredients
Water, C12-15 Alkyl Benzoate, Butylene Glycol, VP/Eicosene Copolymer, Sodium Cetearyl Sulfate, Methyl Glucose Sesquistearate, Glyceryl Stearate, Cetearyl Alcohol, Dimethicone, Picea Albies Wood Extract, Aloe Barbadensis (Aloe Vera) Leaf Juice, Tocopheryl Acetate, Retinyl Palmitate, Sodium Ascorbyl Phosphate, Tocopherol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Bentonite, Sodium Polyacrylamide, Disodium EDTA, Titanium Dioxide, Sodium Hydroxide, Phenoxyethanol, Chlorphenesin, Ethylhexylglycerin.
Polargel® is a registered trademark of AMCOL International Corp.
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2016 CVS/pharmacy CVS.com® 1-800-SHOP CVSMade in the U.S.A. of U.S. and imported components
V-32991 M050899CP FP100
CVS® Quality
Money Back Guarantee -
Principal Display Panel - Carton Label
SKIN+
PHARMACY®ADVANCED
SUN
THERAPYBROAD SPECTRUM
SPF 30 SUN LOTIONUVA/UVB protection
with Polargel® UV TechnologySunscreen with antioxidant
Vitamins A, C & E for protection
against free radicals- ♦
- Polargel® UV Technology allows
lower sunscreen actives &
optimizes performance - ♦
- with Aloe Vera to help keep
skin moisturized - ♦
- water resistant (80 minutes)
- ♦
- paraben, oil & fragrance free
DERMATOLOGIST TESTED
exclusively at
CVS/pharmacy
Actual Product Size
on Side Panel6 FL OZ (180 mL)
-
Principal Display Panel - Tube Label
SKIN+PHARMACY®
ADVANCED
SUN
THERAPYBROAD SPECTRUM
SPF 30 SUN LOTIONUVA/UVB protection
with Polargel® UV TechnologySunscreen with antioxidant
Vitamins A, C & E for protection
against free radicals- ♦
- Polargel® UV Technology allows
lower sunscreen actives & optimizes
performance - ♦
- with Aloe Vera to help keep
skin moisturized - ♦
- water resistant (80 minutes)
- ♦
- paraben, oil &
fragrance free
DERMATOLOGIST TESTED
exclusively at
CVS/pharmacy6 FL OZ (180 mL)
-
INGREDIENTS AND APPEARANCE
SKIN PHARMACY ADVANCED SUN THERAPY BROAD SPECTRUM SPF 30 SUN
homosalate, octisalate, avobenzone, oxybenzone, octocrylene liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-078 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 40 mg in 1 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 40 mg in 1 mL Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 mL Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 30 mg in 1 mL Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SODIUM CETOSTEARYL SULFATE (UNII: 7ZBS06BH4B) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) BENTONITE (UNII: A3N5ZCN45C) CHLORPHENESIN (UNII: I670DAL4SZ) PICEA ABIES WOOD (UNII: 72GZ8K8996) ALOE VERA LEAF (UNII: ZY81Z83H0X) SODIUM HYDROXIDE (UNII: 55X04QC32I) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-078-01 1 in 1 CARTON 04/01/2016 1 180 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 04/01/2016 Labeler - CVS Health (062312574) Registrant - AMCOL Health & Beauty Solutions, Inc.DBA (872684803) Establishment Name Address ID/FEI Business Operations AMCOL Health & Beauty Solutions, Inc. DBA 872684803 ANALYSIS(69842-078) , MANUFACTURE(69842-078) , LABEL(69842-078) , PACK(69842-078)