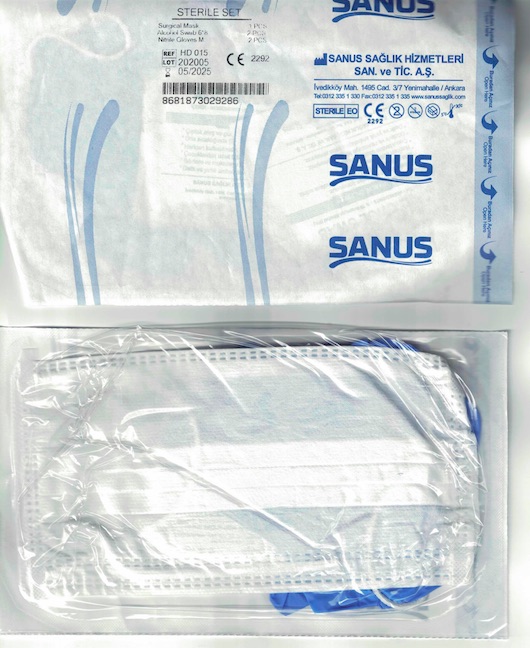
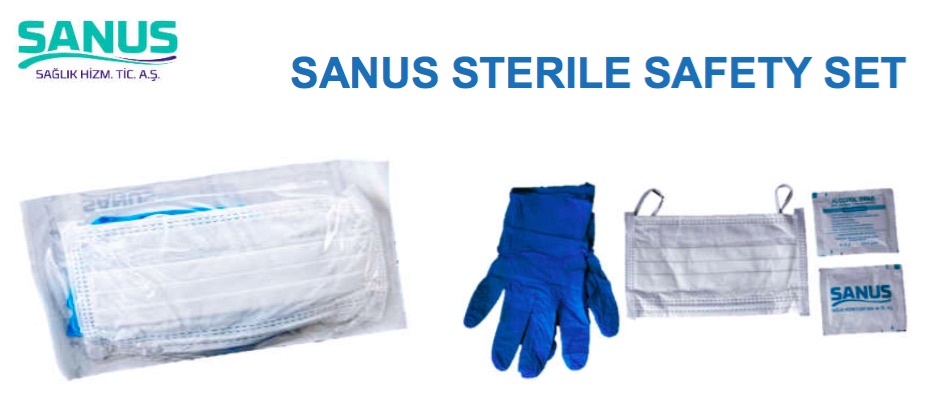
Label: SANUS STERILE SAFETY SET- sterile safety set kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 76557-002-01, 79764-000-01 - Packager: SANUS SAGLIK HIZMETLERI SANAYI VE TICARET ANONIM SIRKETI
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 28, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Directions (Swab)
- Warnings
- Inactive Ingredients
- INDICATIONS & USAGE
- Keep out of reach of children
- Purpose (Swab)
- Sterile Set
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SANUS STERILE SAFETY SET
sterile safety set kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79764-000 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79764-000-01 1 in 1 PACKAGE, COMBINATION; Type 1: Convenience Kit of Co-Package 07/28/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 Part 2 2 Part 3 2 PATCH 2 mL Part 1 of 3 RORO HEALTHCARE DISPOSABLE FACE MASK RORO MEDICAL DISPOSABLE FACE MASK
face mask (except n95 respirator) for general public/healthcare personnel per iie guidance clothProduct Information Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength POLYPROPYLENE (30000 MW) (UNII: T71QXI2O62) 66 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final C48623 07/28/2020 Part 2 of 3 DISPOSABLE NITRILE EXAMINATION GLOVE
polymer patient examination glove clothProduct Information Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength ACRYLONITRILE (UNII: MP1U0D42PE) 100 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final LZA 07/28/2020 Part 3 of 3 JUNIPER CLEAN ALCOHOL SWAB
70% alcohol swab swabProduct Information Item Code (Source) NDC:76557-002 Route of Administration TOPICAL, CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76557-002-01 1 mL in 1 PATCH; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 07/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 07/28/2020 Labeler - SANUS SAGLIK HIZMETLERI SANAYI VE TICARET ANONIM SIRKETI (533144738)