Label: KYMRIAH- tisagenlecleucel injection, suspension
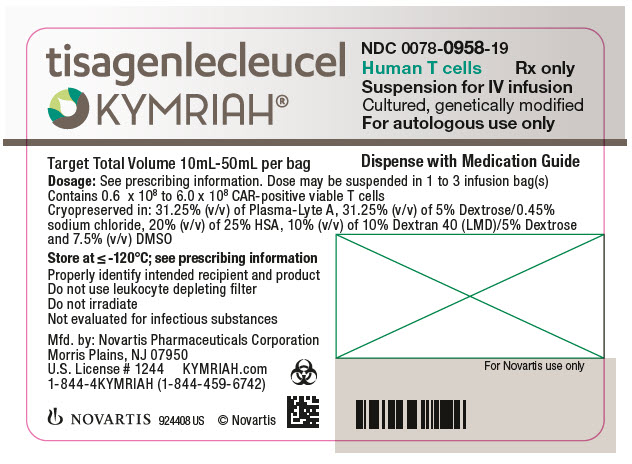
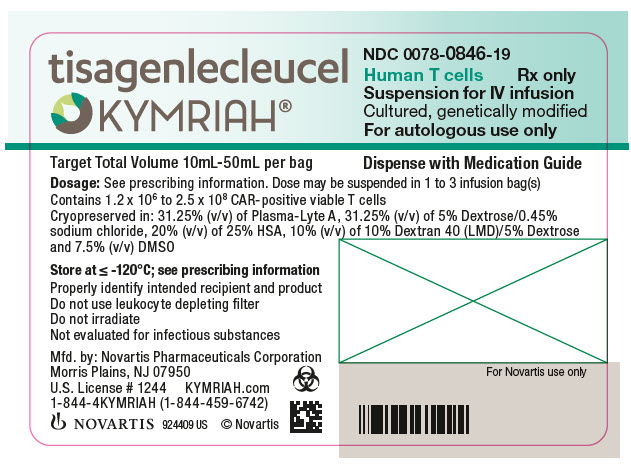
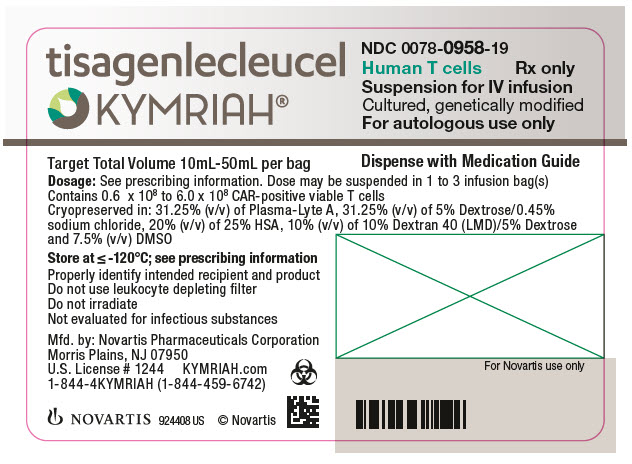
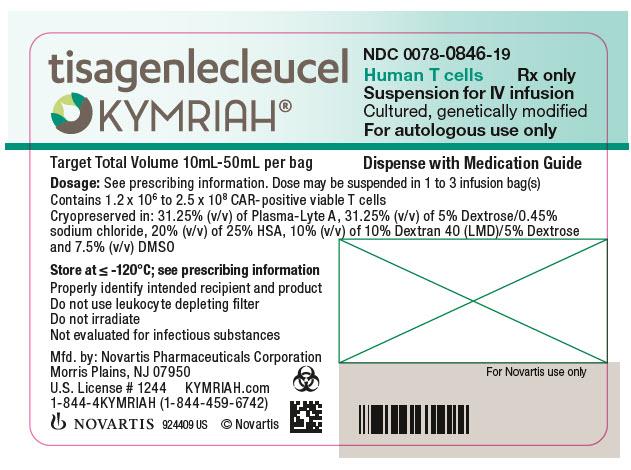
- NDC Code(s): 0078-0846-19, 0078-0958-19
- Packager: Novartis Pharmaceuticals Corporation
- Category: CELLULAR THERAPY
Drug Label Information
Updated August 1, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use KYMRIAH safely and effectively. See full prescribing information for KYMRIAH.
KYMRIAH® (tisagenlecleucel) suspension for intravenous infusion
Initial U.S. Approval: 2017WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGICAL TOXICITIES
See full prescribing information for complete boxed warning.
- Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients receiving KYMRIAH. Do not administer KYMRIAH to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids. (2.3, 2.4, 5.1)
- Neurological toxicities, which may be severe or life-threatening, can occur following treatment with KYMRIAH, including concurrently with CRS. Monitor for neurological events after treatment with KYMRIAH. Provide supportive care as needed. (5.2)
- KYMRIAH is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the KYMRIAH REMS. (5.3)
RECENT MAJOR CHANGES
Indications and Usage, Adult Relapsed or Refractory (r/r) Follicular Lymphoma (FL) (1.3) 5/2022 Dosage and Administration Adult Relapsed or Refractory (r/r) Follicular Lymphoma (FL) (2.2, 2.3) 5/2022 Management of Severe Adverse Reactions (2.4) 5/2022 Warnings and Precautions (5.1, 5.2, 5.4, 5.6, 5.7, 5.8) 5/2022 INDICATIONS AND USAGE
KYMRIAH is a CD19-directed genetically modified autologous T-cell immunotherapy indicated for the treatment of:
- Patients up to 25 years of age with B-cell precursor acute lymphoblastic leukemia (ALL) that is refractory or in second or later relapse. (1.1)
- Adult patients with relapsed or refractory (r/r) large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high grade B-cell lymphoma and DLBCL arising from follicular lymphoma.
Limitations of Use: KYMRIAH is not indicated for treatment of patients with primary central nervous system lymphoma. (1.2) - Adult patients with relapsed or refractory follicular lymphoma (FL) after two or more lines of systemic therapy. This indication is approved under accelerated approval based on response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s). (1.3)
DOSAGE AND ADMINISTRATION
For autologous use only. For intravenous use only.
- Administer a lymphodepleting regimen if needed before infusion of KYMRIAH. (2.3)
- Do NOT use a leukodepleting filter. (2.3)
- Verify the patient’s identity prior to infusion. (2.3)
- Premedicate with acetaminophen and an H1-antihistamine. (2.3)
- Confirm availability of tocilizumab prior to infusion. (2.3, 5.1)
- Dosing of KYMRIAH is based on the number of chimeric antigen receptor (CAR)-positive viable T cells.
-
Pediatric and Young Adult B-cell ALL (up to 25 years of age)
• For patients 50 kg or less, administer 0.2 to 5.0 x 106 CAR-positive viable T cells per kg body weight intravenously. (2.1)
• For patients above 50 kg, administer 0.1 to 2.5 x 108 total CAR-positive viable T cells (non-weight based) intravenously. (2.1)
-
Adult Relapsed or Refractory Diffuse Large B-cell Lymphoma and Follicular Lymphoma
• Administer 0.6 to 6.0 x 108 CAR-positive viable T cells intravenously. (2.2)
DOSAGE FORMS AND STRENGTHS
- Pediatric and Young Adult B-cell ALL (up to 25 years of age)
A single dose of KYMRIAH contains 0.2 to 5.0 x 106 CAR-positive viable T cells per kg of body weight for patients 50 kg or less, or 0.1 to 2.5 x 108 CAR-positive viable T cells for patients more than 50 kg, suspended in one to three patient-specific infusion bag(s) for intravenous infusion. (3)
- Adult Relapsed or Refractory Diffuse Large B-cell Lymphoma and Follicular Lymphoma
A single dose of KYMRIAH contains 0.6 to 6.0 x 108 CAR-positive viable T cells suspended in one to three patient-specific infusion bag(s) for intravenous infusion. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
-
Hypersensitivity Reactions: Monitor for hypersensitivity reactions during infusion. (5.5)
-
Serious Infections: Monitor patients for signs and symptoms of infection; treat appropriately. (5.6)
-
Prolonged Cytopenias: Patients may exhibit ≥ Grade 3 cytopenias for several weeks following KYMRIAH infusion. Prolonged neutropenia has been associated with increased risk of infection. (5.7)
-
Hypogammaglobulinemia: Monitor and provide replacement therapy until resolution. Assess immunoglobulin levels in newborns of mothers treated with KYMRIAH. (5.8)
-
Secondary Malignancies: In the event that a secondary malignancy occurs after treatment with KYMRIAH, contact Novartis Pharmaceuticals Corporation at 1-844-4KYMRIAH. (5.9)
- Effects on Ability to Drive and Use Machines: Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, for at least 8 weeks after receiving KYMRIAH. (5.10)
ADVERSE REACTIONS
Pediatric and Young Adult B-cell ALL (up to 25 years of age): The most common adverse reactions (incidence greater than 20%) are cytokine release syndrome, infections-pathogen unspecified, hypogammaglobulinemia, fever, decreased appetite, viral infectious disorders, headache, febrile neutropenia, hemorrhage, musculoskeletal pain, vomiting, encephalopathy, diarrhea, hypotension, cough, nausea, bacterial infectious disorders, pain, hypoxia, tachycardia, edema, fatigue, and acute kidney injury. (6.1)
Adult Relapsed or Refractory Diffuse Large B-cell Lymphoma: The most common adverse reactions (incidence greater than 20%) are CRS, infections-pathogen unspecified, fever, diarrhea, nausea, fatigue, hypotension, edema, hemorrhage, dyspnea, and headache. (6.1)
Adult Relapsed or Refractory Follicular Lymphoma: The most common adverse reactions (incidence greater than 20%) are cytokine release syndrome, infections-pathogens unspecified, fatigue, musculoskeletal pain, headache, and diarrhea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 5/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGICAL TOXICITIES
1 INDICATIONS AND USAGE
1.1 Pediatric and Young Adult Relapsed or Refractory (r/r) B-cell Acute Lymphoblastic Leukemia (ALL)
1.2 Adult Relapsed or Refractory (r/r) Diffuse Large B-cell Lymphoma (DLBCL)
1.3 Adult Relapsed or Refractory (r/r) Follicular Lymphoma (FL)
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in Pediatric and Young Adult Relapsed or Refractory (r/r) B-cell Acute Lymphoblastic Leukemia (ALL)
2.2 Dosage in Adult Relapsed or Refractory (r/r) Diffuse Large B-cell Lymphoma (DLBCL) and Follicular Lymphoma (FL)
2.3 Administration
2.4 Management of Severe Adverse Reactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cytokine Release Syndrome (CRS)
5.2 Neurological Toxicities
5.3 KYMRIAH REMS to Mitigate Cytokine Release Syndrome and Neurological Toxicities
5.4 Hemophagocytic Lymphohistiocytosis (HLH)/Macrophage Activation Syndrome (MAS)
5.5 Hypersensitivity Reactions
5.6 Serious Infections
5.7 Prolonged Cytopenias
5.8 Hypogammaglobulinemia
5.9 Secondary Malignancies
5.10 Effects on Ability to Drive and Use Machines
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
6.3 Immunogenicity
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics/Cellular Kinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Relapsed or Refractory (r/r) B-cell Acute Lymphoblastic Leukemia (ALL)
14.2 Adult Relapsed or Refractory (r/r) Diffuse Large B-cell Lymphoma (DLBCL)
14.3 Adult Relapsed or Refractory (r/r) Follicular Lymphoma (FL)
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGICAL TOXICITIES
- Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients receiving KYMRIAH. Do not administer KYMRIAH to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids [see Dosage and Administration (2.3, 2.4), Warnings and Precautions (5.1)].
-
Neurological toxicities, which may be severe or life-threatening, can occur following treatment with KYMRIAH, including concurrently with CRS. Monitor for neurological events after treatment with KYMRIAH. Provide supportive care as needed [see Warnings and Precautions (5.2)].
- KYMRIAH is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the KYMRIAH REMS [see Warnings and Precautions (5.3)].
-
1 INDICATIONS AND USAGE
KYMRIAH is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of:
1.1 Pediatric and Young Adult Relapsed or Refractory (r/r) B-cell Acute Lymphoblastic Leukemia (ALL)
Patients up to 25 years of age with B-cell precursor acute lymphoblastic leukemia (ALL) that is refractory or in second or later relapse.
1.2 Adult Relapsed or Refractory (r/r) Diffuse Large B-cell Lymphoma (DLBCL)
Adult patients with relapsed or refractory (r/r) large B-cell lymphoma after two or more lines of systemic therapy including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high grade B-cell lymphoma and DLBCL arising from follicular lymphoma.
Limitation of Use: KYMRIAH is not indicated for treatment of patients with primary central nervous system lymphoma.
1.3 Adult Relapsed or Refractory (r/r) Follicular Lymphoma (FL)
Adult patients with relapsed or refractory (r/r) follicular lymphoma (FL) after two or more lines of systemic therapy.
This indication is approved under accelerated approval based on response rate and duration of response [see Clinical Studies (14.3)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s).
-
2 DOSAGE AND ADMINISTRATION
For autologous use only. For intravenous use only.
2.1 Dosage in Pediatric and Young Adult Relapsed or Refractory (r/r) B-cell Acute Lymphoblastic Leukemia (ALL)
KYMRIAH is provided as a single-dose for infusion containing a suspension of chimeric antigen receptor (CAR)-positive viable T cells.
Based on the patient weight reported at the time of leukapheresis:
- Patients 50 kg or less: administer 0.2 to 5.0 x 106 CAR-positive viable T cells per kg body weight.
- Patients above 50 kg: administer 0.1 to 2.5 x 108 CAR-positive viable T cells.
2.2 Dosage in Adult Relapsed or Refractory (r/r) Diffuse Large B-cell Lymphoma (DLBCL) and Follicular Lymphoma (FL)
KYMRIAH is provided as a single-dose for infusion containing a suspension of chimeric antigen receptor (CAR)-positive viable T cells.
- For adult patients: administer 0.6 to 6.0 x 108 CAR-positive viable T cells.
2.3 Administration
Preparing Patient for KYMRIAH Administration with Lymphodepletion
- Confirm availability of KYMRIAH prior to starting the lymphodepleting regimen.
Pediatric and Young Adult Relapsed or Refractory (r/r) B-cell Acute Lymphoblastic Leukemia (ALL)
- Lymphodepleting chemotherapy: Fludarabine (30 mg/m2 intravenously daily for 4 days) and cyclophosphamide (500 mg/m2 intravenously daily for 2 days starting with the first dose of fludarabine).
- Infuse KYMRIAH 2 to 14 days after completion of the lymphodepleting chemotherapy.
Adult Relapsed or Refractory (r/r) Diffuse Large B-cell Lymphoma (DLBCL) and r/r Follicular Lymphoma (FL)
• Lymphodepleting chemotherapy: Fludarabine (25 mg/m2 intravenously daily for 3 days) and cyclophosphamide (250 mg/m2 intravenously daily for 3 days starting with the first dose of fludarabine).
• Alternate lymphodepleting chemotherapy: bendamustine 90 mg/m2 intravenously daily for 2 days if a patient experienced a previous Grade 4 hemorrhagic cystitis with cyclophosphamide or demonstrates resistance to a previous cyclophosphamide containing regimen.
• Infuse KYMRIAH 2 to 11 days (r/r DLBCL) or 2 to 6 days (r/r FL) after completion of the lymphodepleting chemotherapy.
• Lymphodepleting chemotherapy may be omitted if a patient is experiencing significant cytopenia, e.g., white blood cell (WBC) count is less than 1 x 109/L within 1 week prior to KYMRIAH infusion.
Preparation of KYMRIAH for Infusion and Administration
Delay the infusion of KYMRIAH if a patient has unresolved serious adverse reactions (including pulmonary reactions, cardiac reactions, or hypotension) from preceding chemotherapies, active uncontrolled infection, active graft versus host disease (GVHD), or worsening of leukemia burden following lymphodepleting chemotherapy [see Warnings and Precautions (5.1)].
A KYMRIAH dose may be contained in one to three cryopreserved patient specific infusion bag(s). Verify the number of bags received for the dose of KYMRIAH with the Certificate of Conformance (CoC) and Certificate of Analysis (CoA). Coordinate the timing of thaw of KYMRIAH and infusion in the following manner. Confirm the infusion time in advance, and adjust the start time for thaw so that KYMRIAH is available for infusion when the recipient is ready. If more than one bag has been received for the treatment dose, thaw 1 bag at a time. Wait to thaw/infuse the next bag until it is determined that the previous bag is safely administered.
Preparation of KYMRIAH for Infusion
1. Ensure tocilizumab and emergency equipment are available prior to infusion and during the recovery period.
2. Premedicate patient with acetaminophen and diphenhydramine or another H1-antihistamine approximately 30 to 60 minutes prior to KYMRIAH infusion. Avoid prophylactic use of systemic corticosteroids, as it may interfere with the activity of KYMRIAH.
3. Confirm patient identity: Prior to KYMRIAH preparation, match the patient's identity with the patient identifiers on each KYMRIAH infusion bag(s). KYMRIAH is for autologous use only. Employ universal precautions to avoid potential transmission of infectious diseases when handling the product.
Note: The patient identifier number may be preceded by the letters DIN or Aph ID.
Figure 1. KYMRIAH Infusion Bag
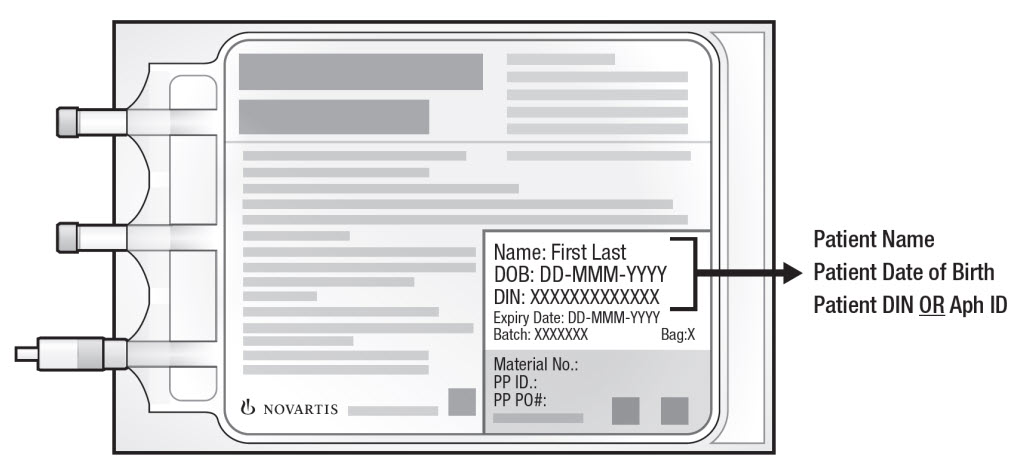
4. Inspect the infusion bag(s) for any breaks or cracks prior to thawing. If a bag is compromised, do not infuse the contents. Call Novartis at 1-844-4KYMRIAH.
5. Place the infusion bag inside a second, sterile bag in case of a leak and to protect ports from contamination.
6. Thaw each infusion bag one at a time at 37°C using either a water bath or dry thaw method until there is no visible ice in the infusion bag. Remove bag from thawing device immediately; do not store product bag at 37°C. Once the infusion bag has been thawed and is at room temperature (20°C to 25°C), it should be infused within 30 minutes. Do not wash, spin down, and/or resuspend KYMRIAH in new media prior to infusion.
7. Inspect the contents of the thawed infusion bag for any visible cell clumps. If visible cell clumps remain, gently mix the contents of the bag. Small clumps of cellular material should disperse with gentle manual mixing. Do not infuse KYMRIAH if clumps are not dispersed, the infusion bag is damaged or leaking, or otherwise appears to be compromised. Call Novartis at 1-844-4KYMRIAH.
Administration
8. Confirm the patient’s identity with the patient identifiers on the infusion bag.
9. Administer KYMRIAH as an intravenous infusion at 10 mL to 20 mL per minute, adjusted as appropriate for smaller children and smaller volumes. The volume in the infusion bag ranges from 10 mL to 50 mL. Do NOT use a leukocyte-depleting filter. If more than one bag is being infused for the treatment dose, wait to thaw/infuse the next bag until it is determined that the previous bag is safely administered.
- Prime the tubing prior to infusion with sodium chloride 9 mg/mL (0.9%) solution for injection.
- Infuse all contents of the infusion bag.
- Rinse the infusion bag with 10 mL to 30 mL sodium chloride 9 mg/mL (0.9%) solution for injection while maintaining a closed tubing system to assure as many cells as possible are infused into the patient.
- Cells from all the bag(s) must be infused to complete a single dose.
KYMRIAH contains human cells genetically modified with a lentivirus. Follow local biosafety guidelines applicable for handling and disposal of such products.
Monitoring
- Administer KYMRIAH at a REMS-certified healthcare facility.
- Monitor patients 2-3 times during the first week following KYMRIAH infusion at the certified healthcare facility for signs and symptoms of CRS and neurologic toxicities [see Warnings and Precautions (5.1, 5.2)].
- Instruct patients to remain within proximity of the certified healthcare facility for at least 4 weeks following infusion.
- Instruct patients to refrain from driving or hazardous activities for at least 8 weeks following infusion.
2.4 Management of Severe Adverse Reactions
Cytokine Release Syndrome
Identify cytokine release syndrome (CRS) based on clinical presentation [see Warnings and Precautions (5.1)]. Evaluate for and treat other causes of fever, hypoxia, and hypotension. If CRS is suspected, manage according to the recommendations in Table 1 (Lee et al 2014). Alternative CRS management strategies may be implemented based on appropriate institutional or academic guidelines.
Table 1. CRS Grading and Management Guidance aLee et al. 2014.
bSantomasso et al. 2021.
cRefer to tocilizumab Prescribing Information for details.
dAlternative therapy includes anti-cytokine and anti-T cell therapies as per institutional policy and published guidelines such as (but not limited to) anakinra, siltuximab, ruxolitinib, cyclophosphamide, IVIG and ATG.CRS Grade a Symptomatic treatment Tocilizumab Corticosteroids Grade 1
Mild symptoms requiring symptomatic treatment only (e.g., low grade fever, fatigue, anorexia, etc.)Exclude other causes (e.g., infection) and treat specific symptoms (e.g., with antipyretics, antiemetics, analgesics, etc.) In patients with persistent (> 3 days) or refractory fever, consider managing as Grade 2 CRSb. Not applicable Grade 2
Symptoms require and respond to moderate intervention Oxygen requirement < 40% or Hypotension responsive to fluids or low dose of one vasopressor or Grade 2 organ toxicityAntipyretics, oxygen, intravenous fluids and/or low dose vasopressors as needed. Administer tocilizumabc intravenously over 1 hour:
- 8 mg/kg (max. 800 mg) if body weight ≥ 30 kg
- 12 mg/kg if body weight < 30 kg
If no improvement after first dose, repeat every 8 hours (limit to a maximum of 3 dosages in 24 hours period; maximum total of 4 doses).If no improvement within 24 hours of tocilizumab, administer a daily dose of 2 mg/kg/day methylprednisolone intravenously (or equivalent) until vasopressor and oxygen no longer needed, then taper.
If not improving, manage as appropriate grade below.Grade 3
Symptoms require and respond to aggressive intervention Oxygen requirement ≥ 40%
or
Hypotension requiring high dose or multiple vasopressors or Grade 3 organ toxicity or Grade 4 transaminitisHigh-flow oxygen
Intravenous fluids, and high-dose or multiple vasopressors
Treat other organ toxicities as per local guidelines.Per Grade 2
If not improving, consider alternative therapyd.Per Grade 2
If not improving, manage as Grade 4.Grade 4
Life-threatening symptoms Requirement for ventilator support or Grade 4 organ toxicity (excluding transaminitis)Mechanical ventilation Intravenous fluids and high-dose vasopressor(s) Treat other organ toxicities as per local guidelines. Per Grade 2
If not improving, consider alternative therapyd.Administer methylprednisolone 1,000 mg intravenously one to two times per day for 3 days.
If not improving, consider methylprednisolone 1,000 mg intravenously two to three times a day or alternate therapyd.
Continue corticosteroids until improvement to Grade 1, and then taper as clinically appropriate.Neurologic Toxicities
Patients should be monitored for neurologic toxicities, including ICANS, following KYMRIAH infusion, particularly during and after resolution of CRS. Identify neurologic toxicities based on clinical presentation. Evaluate for and treat other causes of neurological symptoms. Consider non-sedating seizure prophylaxis (e.g., levetiracetam) for patients at higher risk of seizure, such as those with seizure history, CNS disease, concerning EEG findings, or neoplastic brain lesions. If neurologic toxicity is suspected, manage according to the recommendations in Table 2. Alternative neurologic toxicities management strategies may be implemented based on appropriate institutional, academic, or consensus guidelines.
Table 2: Guidance for Grading and Management of ICANS aASTCT criteria for grading NT (Lee et al. 2019); NCI CTCAE criteria for grading NT used in clinical trials.
bICE Assessment Tool: (1) Orientation: orientation to year, month, city, and hospital: 4 points. (2) Naming: ability to name three objects (e.g., point to clock, pen, and button): 3 points. (3) Following commands: ability to follow simple commands (e.g., show me 2 fingers or close your eyes and stick out your tongue): 1 point. (4) Writing: ability to write a standard sentence (e.g., Our national bird is the bald eagle): 1 point. (5) Attention: ability to count backward from 100 by 10: 1 point.
cSantomasso et. al. 2021.
dAlternate therapy may include anakinra, siltuximab, ruxolitinib, cyclophosphamide, antithymocyte globulin, or intrathecal hydrocortisone (50 mg) plus methotrexate (12 mg).
*A patient with an ICE score of 0 may be classified as Grade 3 ICANS if awake with global aphasia, but a patient with an ICE score of 0 may be classified as Grade 4 ICANS if unarousable.ICANS Gradea No concurrent CRS Concurrent CRS Grade 1
ICE scoreb: 7-9 with no depressed level of consciousnessOffer supportive care with intravenous hydration and aspiration precautions. Administer tocilizumab at any grade CRS, as per dosage recommendation in Table 1. Caution with repeated tocilizumab doses in patients with ICANS. Consider adding corticosteroids to tocilizumab past the first dose c. Grade 2
ICE scoreb: 3-6 and/or Mild somnolence awaking to voiceSupportive care as above.
Consider dexamethasone 10 mg intravenously every 6-12 hours or methylprednisolone equivalent (1 mg/kg intravenously every 12 hours) until the event is Grade 1 or less. If improving, taper corticosteroids.Administer tocilizumab at any grade CRS, as per dosage recommendation in Table 1. If refractory to tocilizumab past the first dose, administer dexamethasone 10 mg intravenously every 6-12 hours or methylprednisolone equivalent (1 mg/kg intravenously every 12 hours) until the event is Grade 1 or less, then taper corticosteroids. Grade 3
ICE scoreb: 0-2*
and/or Depressed level of consciousness awakening only to tactile stimulus and/or
Any clinical seizure focal or generalized that resolves rapidly or nonconvulsive seizures on EEG that resolve with intervention and/or
Focal or local edema on neuroimagingAdminister dexamethasone 10 mg intravenously every 6-12 hours or methylprednisolone equivalent (1 mg/kg intravenously every 12 hours) Administer tocilizumab at any grade CRS, as per dosage recommendation in Table 1. Administer dexamethasone 10 mg intravenously every 6-12 hours or methylprednisolone equivalent (1 mg/kg intravenously every 12 hours). Continue corticosteroids until the event is Grade 1 or less, then taper corticosteroids. If not improving, manage as Grade 4. Grade 4
ICE scoreb: 0*
(patient is unarousable and unable to perform ICE) and/or Stupor or coma and/or
Life-threatening prolonged seizure (> 5 minutes) or repetitive clinical or electrical seizures without return to baseline in between and/or
Diffuse cerebral edema on neuroimaging, decerebrate or decorticate posturing or papilledema, cranial nerve VI palsy, or Cushing’s triadConsider mechanical ventilation for airway protection.
Administer high-dose methylprednisolone intravenously 1,000 mg one to two times per day for 3 days.
If not improving, consider 1,000 mg of methylprednisolone two to three times per day or alternate therapyd.
Continue corticosteroids until improvement to Grade 1, and then taper as clinically appropriate.
Treat seizures, status epilepticus, and cerebral edema as per institutional guidelines.Administer tocilizumab at any grade CRS, as per dosage recommendation in Table 1.
Administer methylprednisolone 1,000 mg intravenously one to two times per day for 3 days.
If not improving, consider methylprednisolone 1,000 mg intravenously two to three times per day or alternate therapyd.
Continue corticosteroids until improvement to Grade 1, and then taper as clinically appropriate.
Treat seizures, status epilepticus, and cerebral edema as per institutional guidelines. -
3 DOSAGE FORMS AND STRENGTHS
Pediatric and Young Adult r/r B-cell ALL (up to 25 years of age): A single dose of KYMRIAH contains 0.2 to 5.0 x 106 CAR-positive viable T cells per kg of body weight for patients 50 kg and below or 0.1 to 2.5 x 108 CAR-positive viable T cells for patients above 50 kg, suspended in one to three patient-specific infusion bag(s) [see How Supplied/Storage and Handling (16)].
Adult r/r DLBCL and r/r FL: A single dose of KYMRIAH contains 0.6 to 6.0 x 108 CAR-positive viable T cells, which may be suspended in one to three patient-specific infusion bag(s) [see How Supplied/Storage and Handling (16)].
See the CoA for actual cell count. The volume in the infusion bag ranges from 10 mL to 50 mL.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Cytokine Release Syndrome (CRS)
CRS, including fatal or life-threatening reactions, occurred following treatment with KYMRIAH. CRS occurred in 61 (77%) of the 79 pediatric and young adult patients with r/r ALL receiving KYMRIAH, including ≥ Grade 3 CRS (Penn grading system1) occurring in 48% of patients. The median times to onset and resolution of CRS were 3 days (range: 1-22; 1 patient with onset after Day 10) and 8 days (range: 1-36), respectively. Of the 61 patients with CRS, 31 (51%) received tocilizumab. Ten (16%) patients received two doses of tocilizumab and 3 (5%) patients received three doses of tocilizumab; 17 (28%) patients received addition of corticosteroids (e.g., methylprednisolone).
CRS occurred in 85 (74%) of the 115 adult patients with r/r DLBCL receiving KYMRIAH, including ≥ Grade 3 CRS (Penn grading system1) occurring in 23% of patients. The median times to onset and resolution of CRS were 3 days (range: 1-51; 1 patient with onset after Day 10) and 7 days (range: 2-30), respectively. Of the 85 patients with CRS, 19 (22%) received systemic tocilizumab or corticosteroids. Seven (8%) patients received a single dose of tocilizumab and 11 (13%) patients received two doses of tocilizumab; 11 (13%) patients received corticosteroids in addition to tocilizumab. One patient received corticosteroids for CRS without concomitant tocilizumab, and two patients received corticosteroids for persistent neurotoxicity after resolution of CRS.
CRS occurred in 51 (53%) of the 97 adult patients with r/r FL receiving KYMRIAH; all were Grade 1 or 2 CRS (Lee grading system2). The median times to onset and resolution of CRS were 4 days (range: 1-14) and 4 days (range: 1-13), respectively. Of the 51 patients with CRS, 15 (29%) received systemic anticytokine treatment with tocilizumab. Three (6%) patients required 3 dosages of tocilizumab, 4 (8%) patients required 2 dosages and 8 (16%) patients required single dose of tocilizumab. Two (4%) patients received corticosteroids in addition to tocilizumab.
Five deaths occurred within 30 days of KYMRIAH infusion. One patient with r/r ALL died with CRS and progressive leukemia, and one patient had resolving CRS with abdominal compartment syndrome, coagulopathy, and renal failure when an intracranial hemorrhage occurred. Of the 3 r/r DLBCL patients who died within 30 days of infusion, all had CRS in the setting of stable to progressive underlying disease, one of whom developed bowel necrosis.
Among patients with CRS, key manifestations include fever (93% in r/r ALL; 85% in r/r DLBCL; 92% in r/r FL), hypotension (69% in r/r ALL; 45% in r/r DLBCL; 40% in r/r FL), hypoxia (57% in r/r ALL; 35% in r/r DLBCL; 19% in r/r FL), and tachycardia (26% in r/r ALL; 13% in r/r DLBCL; 2% in r/r FL). CRS may be associated with hepatic, renal, and cardiac dysfunction, and coagulopathy.
Delay the infusion of KYMRIAH after lymphodepleting chemotherapy if the patient has unresolved serious adverse reactions from preceding chemotherapies (including pulmonary toxicity, cardiac toxicity, or hypotension), active uncontrolled infection, active graft versus host disease (GVHD), or worsening of leukemia burden [see Dosage and Administration (2.3)].
Risk factors for severe CRS in the pediatric and young adult r/r B-cell ALL population are high pre-infusion tumor burden (greater than 50% blasts in bone marrow), uncontrolled or accelerating tumor burden following lymphodepleting chemotherapy, active infections, and/or inflammatory processes.
Ensure that a minimum of two doses of tocilizumab are available on site prior to infusion of KYMRIAH.
Monitor patients 2-3 times during the first week following KYMRIAH infusion at the REMS-certified healthcare facility for signs and symptoms of CRS. Monitor patients for signs or symptoms of CRS for at least 4 weeks after treatment with KYMRIAH. At the first sign of CRS, immediately evaluate patient for hospitalization and institute treatment with supportive care, tocilizumab and/or corticosteroids as indicated [see Dosage and Administration (2.3, 2.4)].
Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time [see Patient Counseling Information (17)].
5.2 Neurological Toxicities
Neurological toxicities, including severe or life-threatening reactions, occurred following treatment with KYMRIAH. Neurologic toxicities occurred in 56 (71%) of the 79 patients with r/r ALL, including ≥ Grade 3 in 22%. The median times to the first event and duration were 6 days from infusion (range: 1-301) and 7 days, respectively.
Neurologic toxicities occurred in 69 (60%) of the 115 patients with r/r DLBCL, including ≥ Grade 3 in 19%. The median times to the first event and duration were 5 days (range: 1-368) and 17 days, respectively.
Neurologic toxicities occurred in 42 (43%) of the 97 patients with r/r FL, including ≥ Grade 3 in 6%. The median times to the first event and duration were 8 days (range: 1-345) and 5 days, respectively.
Among patients who had a neurological toxicity, 84% occurred within 8 weeks following KYMRIAH infusion. Resolution occurred within 3 weeks in 71% of patients with r/r ALL, 50% of patients with r/r DLBCL, and 74% of patients with r/r FL. Encephalopathy lasting up to 70 days was noted.
The onset of neurological toxicity can be concurrent with CRS, following resolution of CRS or in the absence of CRS.
The most common neurological toxicities observed with KYMRIAH include headache (35% in r/r ALL; 21% in r/r DLBCL; 25% in r/r FL), encephalopathy (30% in r/r ALL; 16% in r/r DLBCL; 3% in r/r FL), delirium (19% in r/r ALL; 5% in r/r DLBCL; 1% in r/r FL), anxiety (16% in r/r ALL; 10% in r/r DLBCL; 2% in r/r FL), sleep disorders (11% in r/r ALL; 10% in r/r DLBCL; 6% in r/r FL), dizziness (5% in r/r ALL; 12% in r/r DLBCL; 8% of r/r FL), tremor (8% in r/r ALL; 6% r/r DLBCL; 3% of r/r FL), and peripheral neuropathy (4% in r/r ALL; 12% in r/r DLBCL; 7% in r/r FL). Other manifestations included seizures and aphasia.
Monitor patients 2-3 times during the first week following KYMRIAH infusion at the REMS-certified healthcare facility for signs and symptoms of neurologic toxicities. Rule out other causes of neurological symptoms. Monitor patients for signs or symptoms of neurologic toxicities for at least 4 weeks after infusion and treat promptly. Neurologic toxicity should be managed with supportive care and/or corticosteroids as needed [see Dosage and Administration (2.3)].
Counsel patients to seek immediate medical attention should signs or symptoms of neurologic toxicity occur at any time [see Patient Counseling Information (17)].
5.3 KYMRIAH REMS to Mitigate Cytokine Release Syndrome and Neurological Toxicities
Because of the risk of CRS and neurological toxicities, KYMRIAH is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the KYMRIAH REMS [see Boxed Warning, Warnings and Precautions (5.1, 5.2)]. The required components of the KYMRIAH REMS are:
- Healthcare facilities that dispense and administer KYMRIAH must be enrolled and comply with the REMS requirements.
- Certified healthcare facilities must have on-site, immediate access to tocilizumab, and ensure that a minimum of two doses of tocilizumab are available for each patient for administration within 2 hours after KYMRIAH infusion, if needed for treatment of CRS.
- Certified healthcare facilities must ensure that healthcare providers who prescribe, dispense or administer KYMRIAH are trained about the management of CRS and neurological toxicities.
Further information is available at www.kymriah-rems.com or 1-844-4KYMRIAH.
5.4 Hemophagocytic Lymphohistiocytosis (HLH)/Macrophage Activation Syndrome (MAS)
Hemophagocytic Lymphohistiocytosis (HLH)/Macrophage Activation Syndrome (MAS), which can be life-threatening or fatal, has occurred following treatment with KYMRIAH. HLH was reported in 6% (5/79) of patients with r/r ALL (time to onset ranged from 3 to 18 days) and 2% (2/115) of patients with r/r DLBCL (times to onset were Day 7 and Day 10); all HLH events occurred during ongoing CRS and resolved. One patient (1%) with r/r FL developed HLH > 1 year after receiving KYMRIAH with a fatal outcome. The patient did not have CRS during or immediately preceding HLH. Treatment of HLH should be administered as per institutional standards.
5.5 Hypersensitivity Reactions
Allergic reactions may occur with infusion of KYMRIAH. Serious hypersensitivity reactions, including anaphylaxis, may be due to the DMSO or dextran 40 in KYMRIAH. Observe patients for hypersensitivity reactions during the infusion.
5.6 Serious Infections
Infections, including life-threatening or fatal infections, occurred following treatment with KYMRIAH. Infections occurred in 57 (72%) of the 79 patients with r/r ALL; 38 patients (48%) experienced ≥ Grade 3 infections, including fatal infections in 2 patients (3%).
Infections occurred in 67 (58%) of the 115 patients with r/r DLBCL; 38 patients (33%) experienced ≥ Grade 3 infections, including fatal infection in 1 patient (1%).
Infections occurred in 50 (52%) of the 97 patients with r/r FL; 20 patients (21%) experienced ≥ Grade 3 infections, including fatal infection in 1 patient (1%).
Prior to KYMRIAH infusion, infection prophylaxis should follow local guidelines. Patients with active uncontrolled infection should not start KYMRIAH treatment until the infection is resolved. Monitor patients for signs and symptoms of infection after treatment with KYMRIAH and treat appropriately [see Dosage and Administration (2.3)].
Febrile neutropenia (≥ Grade 3) was also observed in 34% of patients with r/r ALL, 17% of patients with r/r DLBCL, and 13% of patients with r/r FL after KYMRIAH infusion and may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad spectrum antibiotics, fluids and other supportive care as medically indicated.
Viral Reactivation
Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure and death, can occur in patients treated with drugs directed against B cells.
There is no experience with manufacturing KYMRIAH for patients with a positive test for HIV or with active HBV or active HCV. Perform screening for HBV, HCV, and HIV in accordance with clinical guidelines before collection of cells for manufacturing.
5.7 Prolonged Cytopenias
Patients may exhibit cytopenias for several weeks following lymphodepleting chemotherapy and KYMRIAH infusion.
In the ELIANA study (Study 1), ≥ Grade 3 cytopenias not resolved by Day 28 following KYMRIAH treatment included neutropenia (40%), and thrombocytopenia (27%) among 52 responding patients. At 56 days following KYMRIAH, 17% and 12% of responding patients had ≥ Grade 3 neutropenia or thrombocytopenia, respectively.
In the JULIET study (Study 2), ≥ Grade 3 cytopenias not resolved by Day 28 following KYMRIAH treatment included thrombocytopenia (39%) and neutropenia (25%) among 115 treated patients.
In the ELARA study (Study 3), ≥ Grade 3 cytopenias not resolved by Day 28 following KYMRIAH treatment included thrombocytopenia (17%) and neutropenia (16%) among 97 treated patients.
Prolonged neutropenia has been associated with increased risk of infection. Myeloid growth factors, particularly GM-CSF, are not recommended during the first 3 weeks after KYMRIAH infusion or until CRS has resolved.
5.8 Hypogammaglobulinemia
Hypogammaglobulinemia and agammaglobulinemia related to B-cell aplasia can occur in patients after KYMRIAH infusion.
Hypogammaglobulinemia was reported in 53% of patients treated with KYMRIAH for r/r ALL, 17% of patients with r/r DLBCL, and 18% of patients with r/r FL [see Clinical Pharmacology (12.3)].
Monitor immunoglobulin levels after treatment with KYMRIAH and manage using infection precautions, antibiotic prophylaxis, and immunoglobulin replacement standard guidelines.
Immunization with Live Vaccine
The safety of immunization with live vaccines during or following KYMRIAH treatment has not been studied. Vaccination with live vaccines is not recommended for at least 6 weeks prior to the start of lymphodepleting chemotherapy, during KYMRIAH treatment, and until immune recovery following treatment with KYMRIAH.
Pregnant women who have received KYMRIAH may have hypogammaglobulinemia. Assess immunoglobulin levels in newborns of mothers treated with KYMRIAH.
5.9 Secondary Malignancies
Patients treated with KYMRIAH may develop secondary malignancies or recurrence of their cancer. Monitor life-long for secondary malignancies. In the event that a secondary malignancy occurs, contact Novartis Pharmaceuticals Corporation at 1-844-4KYMRIAH to obtain instructions on patient samples to collect for testing.
5.10 Effects on Ability to Drive and Use Machines
Due to the potential for neurological events, including altered mental status or seizures, patients receiving KYMRIAH are at risk for altered or decreased consciousness or coordination in the 8 weeks following KYMRIAH infusion. Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, during this initial period.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in another section of the label:
- Cytokine Release Syndrome [see Warnings and Precautions (5.1)]
- Neurological Toxicities [see Warnings and Precautions (5.2)]
- Hemophagocytic Lymphohistiocytosis (HLH)/Macrophage Activation Syndrome (MAS) [see Warnings and Precautions (5.4)]
- Infections and Febrile Neutropenia [see Warnings and Precautions (5.6)]
- Prolonged Cytopenias [see Warnings and Precautions (5.7)]
- Hypogammaglobulinemia [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described in the WARNINGS AND PRECAUTIONS and in this section reflect exposure to KYMRIAH in three non-randomized, single-arm studies in which 79 pediatric and young adult patients with relapsed/refractory (r/r) B-cell ALL (ELIANA Study), 115 adults with r/r diffuse large B-cell lymphoma (JULIET Study), and 97 adults with r/r follicular lymphoma (ELARA Study) received a single dose of CAR-positive viable T cells.
Pediatric and Young Adult r/r B-cell Acute Lymphoblastic Leukemia (ALL) (up to 25 years of age)
Based on a recommended dose which was weight-based, all 79 patients in the ELIANA study (Study 1) received a single intravenous dose of KYMRIAH [see Clinical Studies (14.1)]. The most common adverse reactions (> 20%) were cytokine release syndrome (77%), infections-pathogen unspecified (57%), hypogammaglobulinemia (53%), fever (42%), decreased appetite (38%), viral infectious disorders (38%), headache (35%), febrile neutropenia (34%), hemorrhage (32%), musculoskeletal pain (32%), vomiting (32%), encephalopathy (30%), bacterial infectious disorders (29%), diarrhea (29%), hypotension (29%), cough (27%), nausea (27%), pain (25%), hypoxia (25%), tachycardia (24%), edema (23%), fatigue (23%), and acute kidney injury (22%).
The adverse reactions with greater or equal to 10% incidence for any Grade are summarized in Table 3.
Table 3. Selected Adverse Reactions Anytime After Infusion (≥ 10%) Following Treatment with KYMRIAH in Pediatric and Young Adult r/r B-cell ALL (N = 79) aTachycardia includes sinus tachycardia and tachycardia.
bAbdominal pain includes abdominal pain, abdominal pain upper.
cPain includes pain and pain in extremity.
dFatigue includes fatigue and malaise.
eEdema includes face edema, fluid overload, generalized edema, localized edema, edema peripheral.
fHypogammaglobulinemia includes hypogammaglobulinemia, immunoglobulins decreased, blood immunoglobulin G decreased, blood immunoglobulin A decreased, blood immunoglobulin M decreased, immunodeficiency, immunodeficiency common variable.
gHyperferritinemia includes serum ferritin increased.
hMusculoskeletal pain includes back pain, bone pain, musculoskeletal chest pain, musculoskeletal pain, myalgia, neck pain, and non-cardiac chest pain.
iHeadache includes headache and migraine.
jEncephalopathy includes encephalopathy, cognitive disorder, confusional state, depressed level of consciousness, disturbance in attention, lethargy, mental status changes, somnolence, memory impairment, and automatism. Encephalopathy is a dominant feature of immune effector cell-associated neurotoxicity syndrome (ICANS), along with other symptoms.
kDelirium includes delirium, agitation, hallucination, hallucination visual, irritability, restlessness.
lSleep disorder includes sleep disorder, insomnia and nightmare.
mAcute kidney injury includes acute kidney injury, anuria, azotemia, renal failure, renal tubular dysfunction, renal tubular necrosis, and blood creatinine increased.
nCough includes cough and productive cough.
oDyspnea includes acute respiratory failure, dyspnea, respiratory distress, and respiratory failure.
pRash includes dermatitis, rash, rash maculo-papular, rash papular, and rash pruritic.
qHemorrhage includes anal hemorrhage, catheter site hemorrhage, cerebral hemorrhage, conjunctival hemorrhage, contusion, cystitis hemorrhagic, disseminated intravascular coagulation, epistaxis, gastrointestinal hemorrhage, gingival bleeding, hemarthrosis, hematemesis, hematuria, hemoptysis, heavy menstrual bleeding, melena, mouth hemorrhage, peritoneal hematoma, petechiae, pharyngeal hemorrhage, purpura, retinal hemorrhage, vaginal hemorrhage.Adverse Reaction All Grades
(%)Grades 3 or Higher
(%)Blood and lymphatic system disorders Febrile neutropenia 34 34 Cardiac disorders Tachycardiaa 24 4 Gastrointestinal disorders Vomiting 32 1 Diarrhea 29 1 Nausea 27 3 Abdominal painb 18 3 Constipation 18 0 General disorders and administration site conditions Fever 42 13 Painc 25 3 Fatigued 23 0 Edemae 23 8 Immune system disorders Cytokine release syndrome 77 48 Hypogammaglobulinemiaf 53 13 Infections and infestations Infections-pathogen unspecified 57 27 Viral infectious disorders 37 22 Bacterial infectious disorders 29 16 Fungal infectious disorders 15 9 Metabolism and nutrition disorders Decreased appetite 38 15 Hypocalcemia 20 6 Hyperferritinemiag 10 3 Musculoskeletal and connective tissue disorders Musculoskeletal painh 32 4 Arthralgia 14 1 Nervous system disorders Headachei 35 3 Encephalopathyj 30 9 Psychiatric disorders Deliriumk 19 4 Anxiety 17 3 Sleep disorderl 11 0 Renal and urinary disorders Acute kidney injurym 22 14 Respiratory, thoracic and mediastinal disorders Coughn 27 0 Hypoxia 25 20 Dyspneao 19 14 Pulmonary edema 15 9 Nasal congestion 11 0 Oropharyngeal pain 10 0 Pleural effusion 10 4 Tachypnea 10 5 Skin and subcutaneous tissue disorders Rashp 18 1 Vascular disorders Hemorrhageq 32 10 Hypotension 29 20 Hypertension 19 5 aCardiac failure includes cardiac failure, cardiac failure congestive, left ventricular dysfunction, right ventricular dysfunction.
bArrhythmia includes cardiac arrest.
cSeizure includes generalized tonic-clonic seizure and seizure.
dPeripheral neuropathy includes hyperasthesia, hypoasthesia, paresthesia.
eSpeech disorder includes aphasia and dysarthria.
fMotor dysfunction includes muscle spasms.Additional important adverse reactions that did not meet the threshold criteria for inclusion in Table 3 were: Blood and lymphatic system disorders: coagulopathy (6%), hemophagocytic lymphohistiocytosis (6%), pancytopenia (3%), Cardiac disorders: cardiac failurea (9%), arrhythmiab (4%) Eye disorders: visual impairment (3%) Gastrointestinal disorders: abdominal distention (4%), ascites (4%), stomatitis (4%), abdominal compartment syndrome (1%), dry mouth (1%) General disorders and administration site conditions: chills (9%), asthenia (4%), influenza-like illness (3%), multiple organ dysfunction syndrome (3%) Immune system disorders: infusion related reaction (6%), graft versus host disease (3%) Investigations: prothrombin time prolonged (4%), fibrin D dimer increased (3%), weight decreased (3%) Metabolism and nutrition disorders: tumor lysis syndrome (6%), hypercalcemia (4%) Nervous system disorders: tremor (8%), seizurec (6%), dizziness (5%), peripheral neuropathyd (4%), speech disordere (3%), motor dysfunctionf (1%), neuralgia (1%) Respiratory, thoracic, and mediastinal disorders: acute respiratory distress syndrome (4%), lung infiltration (1%) Skin and subcutaneous tissue disorders: pruritus (9%), erythema (6%), hyperhidrosis (4%), night sweats (1%) Vascular disorders: capillary leak syndrome (3%), thrombosis (3%), flushing (1%) Laboratory Abnormalities
Table 4. Grade 3 or 4 Laboratory Abnormalities occurring in > 10% of Patients Following Treatment with KYMRIAH in Pediatric and Young Adult r/r B-cell ALL based on CTCAEa (N = 79) aCTCAE = Common Terminology Criteria for Adverse Events version 4.03. Laboratory Abnormality Grade 3 or 4 (%) Hematology Blood fibrinogen decreased 11 Biochemistry Aspartate aminotransferase increased 29 Hypokalemia 28 Alanine aminotransferase increased 22 Hypophosphatemia 20 Hyperbilirubinemia 19 Hyperglycemia 13 All patients experienced neutropenia, anemia and thrombocytopenia. See Table 5 for the incidences of ≥ Grade 3 prolonged thrombocytopenia and prolonged neutropenia in responding patients.
Table 5. Prolonged Cytopenias Following Treatment with KYMRIAH in Pediatric and Young Adult r/r B-cell ALL a≥ Grade 3 observed within 14 days after Day 28 or Day 56 in responding patients. Prolonged Cytopenia N = 52 (%) N = 52 (%) Day 28 Day 56 Prolonged neutropeniaa 40 17 Prolonged thrombocytopeniaa 27 12 Adult r/r Diffuse Large B-cell Lymphoma (DLBCL)
In the JULIET study (Study 2) 115 adults with r/r DLBCL received a single intravenous dose of KYMRIAH [see Clinical Studies (14.2)]. The most common adverse reactions (incidence > 20%) were cytokine release syndrome, infections-pathogen unspecified, fever, diarrhea, nausea, fatigue, hypotension, edema, bleeding episodes, dyspnea, and headache.
The study population characteristics were: median age of 56 years (range: 22-76 years), 80% DLBCL; a median of 3 prior lines of therapy (range: 1-6), 49% had a prior autologous hematopoietic stem cell transplantation, and 32% had received prior radiation therapy. One hundred seven patients (93%) received lymphodepleting chemotherapy prior to KYMRIAH, that included fludarabine (n = 85) or bendamustine (n = 22).
The adverse reactions with greater than or equal to 10% incidence for any Grade are summarized in Table 6 below.
Table 6. Selected Adverse Reactions Anytime After Infusion Reported in ≥ 10% Following Treatment with KYMRIAH in Adult r/r DLBCL (N = 115) aTachycardia includes sinus tachycardia and tachycardia.
bArrhythmia includes atrial fibrillation, cardiac arrest, supraventricular tachycardia, and ventricular extrasystoles.
cAbdominal pain includes abdominal discomfort, abdominal pain, and abdominal pain upper.
dFatigue includes fatigue and malaise.
eEdema includes face edema, fluid overload, fluid retention, generalized edema, localized edema, edema peripheral, peripheral swelling.
fPain includes pain and pain in extremity.
gHypogammaglobulinemia includes blood immunoglobulin G decreased, immunodeficiency, immunoglobulins decreased and hypogammaglobulinemia.
hMusculoskeletal pain includes back pain, flank pain, musculoskeletal chest pain, neck pain, and non-cardiac chest pain.
iHeadache includes headache and migraine.
jEncephalopathy includes cognitive disorder, confusional state, disturbance in attention, lethargy, mental status changes, somnolence, memory impairment, metabolic encephalopathy and thinking abnormal. Encephalopathy is a dominant feature of immune effector cell-associated neurotoxicity syndrome (ICANS), along with other symptoms.
kPeripheral neuropathy includes paraesthesia, hypoaesthesia, hyperaesthesia, peripheral sensory neuropathy, neuropathy peripheral, cranial nerve paralysis, demyelinating polyneuropathy, Horner’s syndrome, polyneuropathy, and sciatica.
lDizziness includes dizziness, presyncope, and syncope.
mSleep disorder includes insomnia and sleep disorder.
nAcute kidney injury includes acute kidney injury, blood creatinine abnormal, and blood creatinine increased.
oDyspnea includes dyspnea, dyspnea exertional, respiratory distress, and respiratory failure.
pCough includes cough, productive cough, and upper-airway cough syndrome.
qRash includes dermatitis, dermatitis acneiform, dermatitis contact, rash, rash maculo-papular, rash papular, and rash pruritic.
rHypotension includes hypotension and orthostatic hypotension.
sHemorrhage includes anal hemorrhage, blood urine present, cerebral hemorrhage, contusion, cystitis hemorrhagic, disseminated intravascular coagulation, duodenal ulcer hemorrhage, epistaxis, eye contusion, gastrointestinal hemorrhage, hematemesis, hematochezia, hematuria, large intestinal hemorrhage, melena, mouth hemorrhage, petechiae, pharyngeal hemorrhage, post procedural hemorrhage, pulmonary hemorrhage, purpura, retinal hemorrhage, traumatic hematoma, tumor hemorrhage, upper gastrointestinal hemorrhage.Adverse Reaction All Grades
(%)Grades 3 or Higher
(%)Blood and lymphatic system disorders Febrile neutropenia 17 17 Cardiac disorders Tachycardiaa 13 3 Arrhythmiab 10 5 Gastrointestinal disorders Diarrhea 31 1 Nausea 29 1 Constipation 17 1 Abdominal painc 10 2 General disorders and administration site conditions Fever 35 5 Fatigued 27 6 Edemae 27 3 Painf 14 3 Chills 12 0 Immune system disorders Cytokine release syndrome 74 23 Hypogammaglobulinemiag 17 6 Infections and infestations Infections-pathogen unspecified 48 26 Bacterial infectious disorders 17 8 Fungal infectious disorders 11 5 Viral infectious disorders 11 2 Investigations Weight decreased 12 4 Metabolism and nutrition disorders Decreased appetite 14 4 Musculoskeletal and connective tissue disorders Arthralgia 14 0 Musculoskeletal painh 13 1 Nervous system disorders Headachei 21 1 Encephalopathyj 16 11 Peripheral neuropathyk 12 3 Dizzinessl 12 2 Psychiatric disorders Anxiety 10 1 Sleep disorderm 10 0 Renal and urinary disorders Acute kidney injuryn 17 6 Respiratory, thoracic and mediastinal disorders Dyspneao 21 6 Coughp 17 0 Skin and subcutaneous tissue disorders Rashq 11 0 Vascular disorders Hypotensionr 25 9 Hemorrhages 22 8 aCardiac failure includes cardiac failure congestive.
bVisual impairment includes vision blurred and visual impairment.
cHyperferritinemia includes serum ferritin increased.
dMotor dysfunction includes muscle spasms, muscle twitching, myoclonus and myopathy.
eTremor includes dyskinesia and tremor.
fSpeech disorder includes speech disorder, aphasia, and dysarthria.
gNeuralgia includes neuralgia and sciatica.
hSeizure includes PTs seizure and status epilepticus.
iAtaxia includes ataxia and dysmetria.
jDelirium includes delirium, agitation, and irritability.
kOropharyngeal pain includes oral pain and oropharyngeal pain.
lPulmonary edema includes acute pulmonary edema and pulmonary edema.
mThrombosis includes deep vein thrombosis, embolism, pulmonary embolism, thrombosis, vena cava thrombosis, and venous thrombosis.Additional important adverse reactions that did not meet the threshold criteria for inclusion in Table 5 were: Blood and lymphatic system disorders: pancytopenia (3%), hemophagocytic lymphohistiocytosis (2%), B-cell aplasia (1%) Cardiac disorders: cardiac failurea (1%) Eye disorders: visual impairmentb (6%) Gastrointestinal disorders: vomiting (9%), stomatitis (6%), dry mouth (5%), abdominal distension (4%), ascites (3%) General disorders and administration site conditions: influenza-like illness (9%), asthenia (7%), multiple organ dysfunction syndrome (3%) Immune system disorders: infusion related reaction (3%) Investigations: fibrin D dimer increased (4%) Metabolism and nutrition disorders: hypocalcemia (5%), hypercalcemia (4%), hyperferritinemiac (4%), tumor lysis syndrome (2%) Musculoskeletal and connective tissue disorders: myalgia (5%) Nervous system disorders: motor dysfunctiond (6%), tremore (6%), speech disorderf (4%), neuralgiag (3%), seizureh (3%), ataxiai (2%), ischemic cerebral infarction (1%) Psychiatric disorders: deliriumj (5%) Respiratory, thoracic, and mediastinal disorders: hypoxia (8%), oropharyngeal paink (8%), pleural effusion (5%), nasal congestion (4%), pulmonary edemal (3%), tachypnea (3%) Skin and subcutaneous tissue disorders: night sweats (5%), pruritus (4%), hyperhidrosis (4%), erythema (2%) Vascular disorders: thrombosism (6%), hypertension (4%), capillary leak syndrome (1%) Laboratory Abnormalities
Table 7. Grade 3 or 4 Laboratory Abnormalities Occurring in > 10% of Patients Following KYMRIAH Infusion in Adult r/r DLBCL Patients Based on CTCAEa N = 115 aCTCAE = Common Terminology Criteria for Adverse Events version 4.03. Laboratory Parameter Grade 3 or 4 (%) Hematology Lymphopenia 95 Neutropenia 82 Leukopenia 78 Anemia 59 Thrombocytopenia 56 Biochemistry Hypophosphatemia 24 Hypokalemia 13 Adult r/r Follicular Lymphoma (FL)
The safety of KYMRIAH was evaluated in the ELARA study (Study 3), a trial that included 97 patients with r/r FL who received a single intravenous dose of KYMRIAH [see Clinical Studies (14.3)]. Patients with a history of CNS disorders or autoimmune disease requiring systemic immunosuppression were ineligible. The median age was 57 years (range: 29 to 73 years), 34% were female, 75% were White, 13% were Asian, and 1% were Black or African American.
The most common adverse reactions (incidence > 20%) were cytokine release syndrome, infections-pathogen unspecified, fatigue, musculoskeletal pain, headache, and diarrhea.
The adverse reactions with greater than or equal to 10% incidence for any Grade are summarized in Table 8 below.
Table 8. Selected Adverse Reactions Anytime After Infusion Reported in ≥ 10% Following Treatment with KYMRIAH in Adult r/r FL (N = 97) aAbdominal pain includes abdominal pain and abdominal pain upper.
bFatigue includes asthenia, fatigue, and malaise.
cHypogammaglobulinemia includes blood immunoglobulin G decreased and hypogammaglobulinemia.
dMusculoskeletal pain includes back pain, bone pain, flank pain, muscle discomfort, musculoskeletal chest pain, musculoskeletal pain, myalgia, neck pain, and non-cardiac chest pain.
eHeadache includes headache and migraine.
fCough includes cough and productive cough.
gRash includes rash, rash maculo-papular, and rash papular.Adverse Reaction All Grades
(%)Grades 3 or Higher
(%)Blood and lymphatic system disorders Febrile neutropenia 13 13 Gastrointestinal disorders Diarrhea 24 2 Nausea 16 2 Constipation 16 0 Abdominal paina 10 1 General disorders and administration site conditions Fatigueb 27 3 Fever 19 1 Immune system disorders Cytokine release syndrome 53 0 Hypogammaglobulinemiac 18 1 Infections and infestations Infections-pathogen unspecified 38 12 Viral infectious disorders 18 5 Musculoskeletal and connective tissue disorders Musculoskeletal paind 25 1 Arthralgia 10 0 Nervous system disorders Headachee 25 2 Respiratory, thoracic and mediastinal disorders Coughf 19 0 Skin and subcutaneous tissue disorders Rashg 10 0 aHemolysis includes hemolysis and hemolytic anemia.
bCoagulopathy includes coagulopathy and international normalized ratio increased.
cTachycardia includes sinus tachycardia.
dArrhythmia includes atrial fibrillation, atrioventricular block first degree, and electrocardiogram QT prolonged.
eVisual impairment includes blindness (preexisting progressive blindness, which initiated prior to start of lymphodepleting chemotherapy, further worsened after KYMRIAH infusion), vision blurred, and visual impairment.
fStomatitis includes mouth ulceration and stomatitis.
gEdema includes edema peripheral, fluid retention, hypervolemia, localized edema, and peripheral swelling.
hPain includes ear pain, pain, and pain in extremity.
iGraft versus host disease includes graft versus host disease in gastrointestinal tract and graft versus host disease in skin.
jDizziness includes dizziness and syncope.
kMotor dysfunction includes dyskinesia, muscle spasms, muscular weakness, musculoskeletal stiffness, and myoclonus.
lPeripheral neuropathy includes dysesthesia, hypoesthesia, neuropathy peripheral, paresthesia, and peripheral sensory neuropathy.
mSleep disorder includes insomnia.
nAcute kidney injury includes acute kidney injury and blood creatinine increased.
oDyspnea includes acute respiratory failure, dyspnea, and dyspnea exertional.
pHypotension includes hypotension and orthostatic hypotension.
qHemorrhage includes blood blister, catheter site hemorrhage, contusion, epistaxis, hematochezia, hematoma, mucosal hemorrhage, oral blood blister, petechiae, and purpura.
rThrombosis includes deep vein thrombosis.Additional important adverse reactions that did not meet the threshold criteria for inclusion in Table 8 were: Blood and lymphatic system disorders: pancytopenia (3%), hemolysisa (2%), coagulopathyb (2%) Cardiac disorders: tachycardiac (2%), arrhythmiad (4%) Eye disorders: visual impairmente (4%) Gastrointestinal disorders: vomiting (9%), stomatitisf (4%), abdominal distension (2%), dry mouth (2%) General disorders and administration site conditions: edemag (9%), painh (8%), chills (6%) Immune system disorders: infusion related reaction (3%), graft versus host diseasei (1%), hemophagocytic lymphohistiocytosis (1%) Infections and infestations: bacterial infectious disorders (7%), fungal infectious disorders (2%) Investigations: weight decreased (7%) Metabolism and nutrition disorders: decreased appetite (8%), tumor lysis syndrome (2%) Nervous system disorders: dizzinessj (8%), motor dysfunctionk (9%), peripheral neuropathyl (7%), immune effector cell-associated neurotoxicity syndrome (4%), encephalopathy (3%), tremor (3%) Psychiatric disorders: sleep disorderm (6%), anxiety (2%), delirium (1%) Renal and urinary disorder: acute kidney injuryn (4%) Respiratory, thoracic, and mediastinal disorders: dyspneao (8%), pleural effusion (6%), oropharyngeal pain (5%), nasal congestion (2%), rhinorrhea (2%) Skin and subcutaneous tissue disorders: pruritus (9%), night sweats (3%), erythema (2%), hyperhidrosis (1%) Vascular disorders: hypotensionp (9%), hemorrhageq (6%), hypertension (5%), thrombosisr (1%) Laboratory Abnormalities
Table 9. Grade 3 or 4 Laboratory Abnormalities Occurring in > 10% of Patients Following KYMRIAH Infusion in Adult r/r FL Patients Based on CTCAEa (N = 97*) aCTCAE = Common Terminology Criteria for Adverse Events version 4.03.
*Evaluable population (n = 91 to 97) for each laboratory value included number of patients who had both baseline (before KYMRIAH infusion) and at least one post-KYMRIAH infusion on-study laboratory value available.Laboratory Abnormality Grade 3 or 4 (%) Hematology Neutropenia 63 Leukopenia 40 Thrombocytopenia 21 Anemia 20 Lymphopenia 19 Biochemistry Hypophosphatemia 12 6.2 Postmarketing Experience
The following adverse reaction has been identified during postapproval use of KYMRIAH. Because this reaction is reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate its frequency or establish a causal relationship to drug exposure.
- Anaphylactic reaction
6.3 Immunogenicity
In clinical studies, humoral immunogenicity of KYMRIAH was measured by determination of anti-murine CAR19 antibodies (anti-mCAR19) in serum pre- and post-administration. The majority of patients, 91% in ELIANA (Study 1), 94% in JULIET (Study 2), and 66% in ELARA (Study 3), tested positive for pre-dose anti-mCAR19 antibodies prior to KYMRIAH infusion. Treatment induced anti-mCAR19 antibodies were detected in 9% and 33% of the patients in JULIET and ELARA, respectively. However, the preexisting and treatment-induced antibodies were not associated with an impact on clinical response and did not have an impact on the initial expansion and persistence of KYMRIAH. Persistence of KYMRIAH was similar between patients with positive post-infusion anti-mCAR19 antibodies compared with patients with negative post-infusion anti-mCAR19 antibodies. There is no evidence that the presence of preexisting and treatment-induced anti-mCAR19 antibodies impact the safety or effectiveness of KYMRIAH.
T cell immunogenicity responses were not observed in r/r ALL, r/r DLBCL, or r/r FL patients.
- Cytokine Release Syndrome [see Warnings and Precautions (5.1)]
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data with KYMRIAH use in pregnant women. No animal reproductive and developmental toxicity studies have been conducted with KYMRIAH to assess whether it can cause fetal harm when administered to a pregnant woman. It is not known if KYMRIAH has the potential to be transferred to the fetus. Based on the mechanism of action, if the transduced cells cross the placenta, they may cause fetal toxicity, including B-cell lymphocytopenia. Therefore, KYMRIAH is not recommended for women who are pregnant, and pregnancy after KYMRIAH administration should be discussed with the treating physician. Report pregnancies to Novartis Pharmaceuticals Corporation at 1-888-669-6682.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of KYMRIAH in human milk, the effect on the breastfed infant, and the effects on milk production. A risk to the breastfed infant cannot be excluded. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for KYMRIAH and any potential adverse effects on the breastfed infant from KYMRIAH or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Pregnancy status of females with reproductive potential should be verified. Sexually-active females of reproductive potential should have a pregnancy test prior to starting treatment with KYMRIAH.
Contraception
See the prescribing information for fludarabine and cyclophosphamide for information on the need for effective contraception in patients who receive the lymphodepleting chemotherapy.
There are insufficient exposure data to provide a recommendation concerning duration of contraception following treatment with KYMRIAH.
Infertility
There are no data on the effect of KYMRIAH on male and female fertility.
8.4 Pediatric Use
The safety and efficacy of KYMRIAH have been established in pediatric patients with r/r B-cell ALL. Use of KYMRIAH is supported by a single-arm trial [see Clinical Studies (14.1)] that included 61 pediatric patients with r/r B-cell precursor ALL in the following age groups: 40 children (ages 2 years to less than 12 years) and 21 adolescents (ages 12 years to less than 17 years). No differences in efficacy or safety were observed between the different age subgroups or in comparison to the young adults in the trial.
The safety and efficacy of KYMRIAH in pediatric patients with r/r DLBCL and r/r FL have not been established.
-
11 DESCRIPTION
KYMRIAH (tisagenlecleucel) is a CD19-directed genetically modified autologous T cell immunotherapy comprised of autologous T cells that are genetically modified using a lentiviral vector to encode an anti-CD19 chimeric antigen receptor (CAR). The CAR is comprised of a murine single-chain antibody fragment (scFv) specific for CD19, followed by a CD8 hinge and transmembrane region that is fused to the intracellular signaling domains for 4-1BB (CD137) and CD3 zeta.
KYMRIAH is prepared from the patient’s peripheral blood mononuclear cells, which are obtained via a standard leukapheresis procedure. The mononuclear cells are enriched for T cells, then transduced with the lentiviral vector containing the anti-CD19 CAR transgene, and activated with anti-CD3/CD28 antibody coated beads. The transduced T cells are expanded in cell culture, washed, and formulated into a suspension, which then is cryopreserved. The product must pass a sterility test before release for shipping as a frozen suspension in a patient-specific infusion bag(s). The product is thawed prior to administration [see Dosage and Administration (2.3), How Supplied/Storage and Handling (16)]. The thawed product is a colorless to slightly yellow suspension of cells.
In addition to T cells, other cell populations, including monocytes, NK cells, and B-cells, may be present. The formulation contains 31.25% (v/v) of Plasma-Lyte A, 31.25% (v/v) of 5% Dextrose/0.45% sodium chloride, 10% Dextran 40 (LMD)/5% Dextrose, 20% (v/v) of 25% Human Serum Albumin (HSA), and 7.5% (v/v) Cryoserv® dimethylsulfoxide (DMSO).
Pediatric and Young Adult r/r B-cell ALL: A single dose of KYMRIAH may contain up to 2.5 x 108 CAR-positive viable T cells provided in one to three patient-specific infusion bag(s). Based on the patient’s weight reported at the time of leukapheresis, one of two possible dose ranges will be prepared for the patient:
- For patients 50 kg or less: 0.2 to 5.0 x 106 CAR-positive viable T cells per kg body weight.
- For patients above 50 kg: 0.1 to 2.5 x 108 CAR-positive viable T cells.
Adult r/r DLBCL and r/r FL: A single dose of KYMRIAH may contain 0.6 to 6.0 x 108 CAR-positive viable T cells provided in one to three patient-specific infusion bag(s).
The actual number of CAR-positive T cells in the product is reported on the Certificate of Analysis (CoA) that is shipped with KYMRIAH. The volume of CAR-positive viable T cells in an infusion bag ranges from 10 mL to 50 mL.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
KYMRIAH is a CD19-directed genetically modified autologous T cell immunotherapy which involves reprogramming a patient’s own T cells with a transgene encoding a chimeric antigen receptor (CAR) to identify and eliminate CD19-expressing malignant and normal cells. The CAR is comprised of a murine single-chain antibody fragment which recognizes CD19 and is fused to intracellular signaling domains from 4-1BB (CD137) and CD3 zeta. The CD3 zeta component is critical for initiating T cell activation and antitumor activity, while 4-1BB enhances the expansion and persistence of KYMRIAH. Upon binding to CD19-expressing cells, the CAR transmits a signal to promote T cell expansion, activation, target cell elimination, and persistence of the KYMRIAH cells.
12.2 Pharmacodynamics
Due to the on-target effect of KYMRIAH, a period of B-cell aplasia is expected.
Among evaluable pediatric and young adult r/r B-cell ALL patients with an ongoing response at Month 24, 33% had no detectable B cells at baseline prior to infusion. At Month 24, 88% had no detectable B cells.
Most adult r/r DLBCL patients had B-cell depletion at baseline prior to infusion due to previous treatment with rituximab. Recovery of B-cell levels were observed with longer follow-up in some of the responding DLBCL patients after KYMRIAH infusion. Among evaluable adult r/r DLBCL and FL patients with an ongoing response at Month 24, all patients had no detectable B cells at baseline prior to infusion or at Month 24.
12.3 Pharmacokinetics/Cellular Kinetics
Following infusion, KYMRIAH exhibited an initial rapid expansion followed by a bi-exponential decline in pediatric and young adult r/r B-cell acute lymphoblastic leukemia (ALL) patients, adult r/r diffuse large B-cell lymphoma patients, and adult r/r follicular lymphoma patients.
A summary of pharmacokinetic parameters of KYMRIAH is provided in Table 10 below.
Table 10. Pharmacokinetic Parameters of KYMRIAH in Pediatric and Young Adult r/r B-cell ALL, Adult r/r DLBCL, and Adult r/r FL Parameter Summary Statistics Pediatric ALL
Responding Patients
N = 62Pediatric ALL
Non-Responding Patients
N = 8r/r DLBCL
Responding Patients
(CR and PR)
N = 34r/r DLBCL Non-
Responding Patients
(SD/PD/
Unknown)
N = 34r/r FL
Responding
Patients
(CR and PR)
N = 77r/r FL
Non-Responding
Patients (SD/PD)
N = 12Cmax (copies/mcg) Geometric mean (CV%), n 34,700 (155.4), 61 20,000 (71.6), 7 5,210 (256.5), 33 6,450 (408.2), 32 6,250 (344), 64 3,000 (1190), 8 Tmax (day) Median [min; max], n 9.91 [0.008; 27], 61 20.0 [0.03; 62.7], 7 9.83 [5.73; 16.8], 33 8.39 [3.04, 27.7], 32 9.94 [2.62, 28.0], 64 13.0 [7.73, 16.0], 8 AUC0-28d (copies/mcg*day) Geometric mean (CV%), n 318,000 (177.8), 61 156,000 (99.4), 6 58,200 (165.1), 30 75,800 (292.3), 25 56,900 (270), 63 20,100 (18100), 7 T½ (day) Geometric mean (CV%), n 16.8 (155.9), 54 2.52 (171.9), 3 45.3 (157.7), 21 13.6 (167.0), 22 44.0 (296), 42 24.4 (180), 6 Description of Pharmacokinetics in Pediatric and Young Adult r/r B-cell ALL (up to 25 years of age)
The Cmax and AUC0-28d were similar between CR/CRi patients compared with non-responding (NR) patients.
KYMRIAH was present in the blood as well as bone marrow and was measurable beyond 2 years. Blood to bone marrow partitioning suggested that KYMRIAH distribution in bone marrow was 44% of that present in blood at Day 28 while at Months 3 and 6 KYMRIAH distributed at 67% and 69%, respectively, indicating high distribution to bone marrow.
Children < 10 years and between 10-18 years of age had similar- to 1.7-fold higher Cmax and AUC0-28d than adults. Due to small sample size and high variability, it is difficult to assess the impact of age on the pharmacokinetics of KYMRIAH.
Description of Pharmacokinetics in Adult r/r DLBCL
The Cmax and AUC0-28d were similar between responding and non-responding (NR) patients.
KYMRIAH was present in adult r/r DLBCL patients up to 18 months in peripheral blood and up to 9 months in the bone marrow for patients having a complete response. The median time of maximal expansion of transgene levels (Tmax) in peripheral blood occurred at 9-10 days in both responding and non-responding patients.
Description of Pharmacokinetics in Adult r/r FL
KYMRIAH has been detected for up to 18 months in peripheral blood and up to 3 months in bone marrow for patients who achieved a response. The median time of maximal expansion of transgene levels (Tmax) in peripheral blood occurred at 10 days in responding and 13 days in non-responding patients.
The blood to bone marrow partitioning in bone marrow was nearly 50% at Month 3 in responding patients.
The geometric mean AUC0-28d value of responders was 183% higher compared to non-responders, while, the geometric mean Cmax value was 108% higher in responders compared to non-responders. Considering the high inter-individual variability, small number of non-responders, overlapping expansion ranges observed between responders and non-responders, the exposure differences should be interpreted with caution.
Tocilizumab and Corticosteroid used for CRS Management
Some patients required tocilizumab and corticosteroids for the management of CRS. KYMRIAH continues to expand and persist following treatment as per the CRS management algorithm (see Table 1). Patients who have higher expansion tended to have higher CRS Grades [see Warnings and Precautions (5.1)].
Pediatric and young adult r/r B-cell ALL patients (N = 28) treated with tocilizumab had 298% and 183% higher KYMRIAH AUC0-28d and Cmax, respectively, as compared to patients (N = 46) who did not receive tocilizumab. In addition, patients who received corticosteroids for CRS management (N = 17) had 280% higher AUC0-28d compared with patients who did not receive corticosteroids (N = 31).
Adult r/r DLBCL patients treated with tocilizumab (N = 18) had 238% (n = 14) and 311% (n = 16) higher KYMRIAH AUC0-28d and Cmax, respectively, as compared to patients (N = 97) who did not receive tocilizumab. In addition, patients who received corticosteroids for CRS management (N = 12) had 104% and 179% higher AUC0-28d and Cmax, respectively, as compared with patients who did not receive corticosteroids (N = 79).
Adult r/r FL patients treated with tocilizumab (N = 14) had 245% (n = 12) and 312% (n = 11) higher KYMRIAH AUC0-28d and Cmax, respectively, as compared to patients (N = 76) who did not receive tocilizumab. Three patients received corticosteroids for CRS management while all other patients received corticosteroids for other reasons, therefore a formal comparison for exposure differences by use of corticosteroids cannot be performed.
Hepatic and renal impairment studies of KYMRIAH were not conducted.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Genotoxicity assays and carcinogenicity assessment in rodent models were not performed for KYMRIAH. In vitro expansion studies with transduced T cells (KYMRIAH) from healthy donors and patients showed no evidence for transformation and/or immortalization of T cells. In vivo studies in immunocompromised mice did not show signs of abnormal cell growth or signs of clonal cell expansion for up to 7 months after cell injection. A genomic insertion site analysis was performed on KYMRIAH products from 14 individual donors (12 patients and 2 healthy volunteers). There was no evidence for preferential integration near genes of concern, or preferential outgrowth of cells harboring integration sites of concern.
No studies on the effects of KYMRIAH on fertility have been conducted.
-
14 CLINICAL STUDIES
14.1 Relapsed or Refractory (r/r) B-cell Acute Lymphoblastic Leukemia (ALL)
The efficacy of KYMRIAH in pediatric and young adults with r/r B-cell precursor ALL was evaluated in an open-label, multicenter single-arm trial (ELIANA, NCT02435849). In total, 107 patients were screened, 88 were enrolled, 68 were treated, and 63 were evaluable for efficacy. Nine percent of the enrolled patients did not receive the product due to manufacturing failure. The 63 evaluable patients included 35 males and 28 females of median age 12 years (range: 3-23 years). Seventy-three percent of patients were White, 10% were Asian, and 17% were of other races. Six (10%) had primary refractory disease, 30 (48%) had one prior stem cell transplantation, 5 patients (8%) had two stem cell transplantations. Treatment consisted of lymphodepleting chemotherapy (fludarabine 30 mg/m2 daily for 4 days and cyclophosphamide 500 mg/m2 daily for 2 days) followed by a single dose of KYMRIAH. Of the 22 patients who had a WBC count < 1000/µL, 20 received lymphodepleting chemotherapy prior to KYMRIAH while 2 received KYMRIAH infusion without lymphodepleting chemotherapy. Fifty-three patients received bridging chemotherapy between time of enrollment and lymphodepleting chemotherapy.
The efficacy of KYMRIAH was established on the basis of complete remission (CR) within 3 months after infusion, the duration of CR, and proportion of patients with CR and minimal residual disease (MRD) < 0.01% by flow cytometry (MRD-negative) (Table 11). Among the 63 infused patients, 52 (83%) achieved CR/CRi, all of which were MRD-negative. With a median follow-up of 4.8 months from response, the median duration of CR/CRi was not reached (range: 1.2 to 14.1+ months). Median time to onset of CR/CRi was 29 days with onset of CR/CRi between 26 and 31 days for 50/52 (96%) responders. The stem cell transplantation rate among those who achieved CR/CRi was 12% (6/52). Table 11 shows the efficacy results from this study.
Table 11. Efficacy Results in Pediatric and Young Adult Patients with r/r B-cell ALL 1CR/CRi was calculated based on all patients who received KYMRIAH and completed at least 3 months follow-up or discontinued earlier prior to the data cut-off. Requires remission status to be maintained for at least 28 days without clinical evidence of relapse.
2The null hypothesis of CR/CRi less than or equal to 20% was rejected.
3CR (complete remission) was defined as less than 5% of blasts in the bone marrow, no evidence of extramedullary disease, and full recovery of peripheral blood counts (platelets greater than 100,000/microliter and absolute neutrophil counts [ANC] greater than 1,000/microliter) without blood transfusion.
4CRi (complete remission with incomplete blood count recovery) was defined as less than 5% of blasts in the bone marrow, no evidence of extramedullary disease, and without full recovery of peripheral blood counts with or without blood transfusion.
5MRD (minimal residual disease) negative was defined as MRD by flow cytometry less than 0.01%.
6The null hypothesis of MRD-negative remission rate less than or equal to 15% was rejected.
7DOR (duration of remission) was defined as time since onset of CR or CRi to relapse or death due to underlying cancer, whichever is earlier, censoring for new cancer therapy including stem cell transplantation (N = 52).
8Not Estimable.Results N = 63 CR/CRi1,2
(95% CI)52 (83%)
(71%, 91%) p < 0.0001CR3 40 (63%) CRi4 12 (19%) CR or CRi with MRD-negative bone marrow5,6
(95% CI)52 (83%)
(71%, 91%) p < 0.0001Duration of Remission7 N = 52 Median (months) Not reached (95% CI) (7.5, NE8) 14.2 Adult Relapsed or Refractory (r/r) Diffuse Large B-cell Lymphoma (DLBCL)
The efficacy and safety of KYMRIAH was evaluated in an open-label, multicenter, single-arm trial (JULIET; NCT02445248). Eligible patients were ≥ 18 years of age with relapsed or refractory DLBCL, who received ≥ 2 lines of chemotherapy, including rituximab and anthracycline, or relapsed following autologous hematopoietic stem cell transplantation (HSCT). The study excluded patients with active central nervous system malignancy, prior allogenic HSCT, an ECOG performance status ≥ 2, a creatinine clearance < 60, alanine aminotransferase > 5 times normal, cardiac ejection fraction < 45%, or absolute lymphocyte concentration less than 300/µL.
Following 2 to 11 days after completion of lymphodepleting (LD) chemotherapy consisting of either fludarabine (25 mg/m2 intravenously daily for 3 days) and cyclophosphamide (250 mg/m2 intravenously daily for 3 days starting with the first dose of fludarabine) or bendamustine (90 mg/m2 intravenously daily for 2 days), KYMRIAH was administered as a single intravenous infusion. Bridging chemotherapy between leukapheresis and LD chemotherapy was permitted to control disease burden. LD chemotherapy could be omitted if the white blood cell count was < 1000 cells/µL. The major efficacy outcome measures were objective response rate per Lugano criteria [2014] as assessed by an independent review committee and duration of response.
Of the 160 patients enrolled, 106 patients received tisagenlecleucel, including 92 patients who received product manufactured in the U.S. and were followed for at least 3 months or discontinued earlier. Eleven out of 160 patients enrolled did not receive tisagenlecleucel due to manufacturing failure. Thirty-eight other patients did not receive tisagenlecleucel, primarily due to death (n = 16), physician decision (n = 16), and adverse events (n = 3).
Of the 92 patients receiving KYMRIAH, 90% received physician’s choice of bridging chemotherapy in the interval between start of screening and KYMRIAH infusion, among whom the median number of bridging chemotherapy regimens was 1 (range: 1 to 5) with 83% of patients receiving ≤ 2 regimens. A retrospectively identified sub-group of 68 patients was evaluable for the major efficacy outcome measures. Patients included in this sub-group had either had no bridging chemotherapy, or had imaging that showed measurable disease after completion of bridging chemotherapy, prior to KYMRIAH infusion. Of the 24 patients not included, 8 had no evidence of disease at baseline prior to KYMRIAH infusion, 15 did not have baseline imaging following bridging chemotherapy, and 1 was excluded because of initial misclassification of a neuroendocrine tumor as DLBCL.
Among the efficacy evaluable population of 68 patients, the baseline characteristics were: median age 56 years (range: 22 to 74 years); 71% male; 90% White, 4% Asian, and 3% Black or African American; 78% had primary DLBCL not otherwise specified (NOS) and 22% had DLBCL following transformation from follicular lymphoma, of whom 17% were identified as high grade; and 44% had undergone prior autologous HSCT. The median number of prior therapies was 3 (range: 1 to 6), 56% had refractory disease and 44% relapsed after their last therapy. Ninety percent of patients received lymphodepleting chemotherapy (66% of patients received fludarabine and 24% received bendamustine) and 10% did not receive any LD chemotherapy. The median time from leukapheresis and cryopreservation to KYMRIAH infusion was 113 days (range: 47 to 196 days). The median dose was 3.5 × 108 CAR-positive viable T cells (range: 1.0 to 5.2 × 108 cells). Seventy-three percent of patients received KYMRIAH in the inpatient setting.
Efficacy was established on the basis of complete response (CR) rate and duration of response (DOR), as determined by an independent review committee (Table 12 and Table 13). The median time to first response to KYMRIAH (CR or PR) was 0.9 months (range: 0.7 to 3.3 months). The median duration of response was not reached. Response durations were longer in patients who achieved CR, as compared to patients with a best response of partial response (PR) (Table 13). Of the 22 patients who experienced a CR, 9 achieved this status by 1 month, 12 more by month 3, and the last by month 6 after KYMRIAH infusion.
Table 12. Response Rates in Relapsed or Refractory DLBCL in the JULIET Study Response Rate N = 68 Overall Response Rate (ORR) (CR+PR), n (%)
(95% CI)
Complete Response Rate n (%)
(95% CI)
Partial Response Rate n (%)
(95% CI)34 (50%)
(37.6%, 62.4%)
22 (32%)
(21.5%, 44.8%)
12 (18%)
(9.5%, 28.8%)Table 13. Duration of Responsea (Months) in Relapsed or Refractory DLBCL in the JULIET Study CR, Complete Response; DOR, Duration of Response: NE, not estimable, PR, partial response.
aAmong all responders. DOR measured from date of first objective response to date of progression or death from relapse.
bKaplan-Meier estimate in months.
cA + sign indicates a censored value.Duration of Response Results Overall DOR for responders (months) N = 34 Median DORa,b NE (95% CI) (5.1, NE) Rangec (0.03+ – 11.3+) Median Follow-up (95% CI)b 9.4 (7.9, 10.8) DOR if BOR is CR N = 22 Median DORa,b NE (95% CI) (10.0, NE) Rangec (1.5+ – 11.3+) DOR if BOR is PR N = 12 Median DORa,b 3.4 (95% CI) (1.0, NE) Rangec (0.03+ – 11.3+) 14.3 Adult Relapsed or Refractory (r/r) Follicular Lymphoma (FL)
The efficacy of KYMRIAH was evaluated in a multicenter, single-arm, open-label trial (ELARA, Study 3; NCT03568461) that included patients who were refractory to or relapsed within 6 months after completion of two or more lines of systemic therapy (including an anti-CD20 antibody and an alkylating agent), relapsed during or within six months after completion of an anti-CD20 antibody maintenance therapy following at least two lines of therapy, or relapsed after autologous hematopoietic stem cell transplant (HSCT). The trial excluded patients with active or serious infections, transformed lymphoma, or other aggressive lymphomas, prior allogeneic HSCT, or disease with active CNS involvement. Following lymphodepleting (LD) chemotherapy, KYMRIAH was administered as a single dose intravenous infusion with a target dose of 0.6 to 6.0 × 108 CAR-positive viable T cells. The median dose administered was 2.06 × 108 CAR-positive viable T-cells (range: 0.1 to 6.0 × 108 CAR-positive viable T cells). The LD chemotherapy regimen consisted of either fludarabine (25 mg/m2 intravenously daily for 3 days) and cyclophosphamide (250 mg/m2 intravenously daily for 3 days starting with the first dose of fludarabine) or bendamustine (90 mg/m2 IV daily for 2 days); bridging chemotherapy between leukapheresis and LD chemotherapy was permitted as needed. Of the 90 patients included in the primary efficacy analysis, 40 patients (45%) were treated with bridging therapies. The most commonly used agents (in ≥ 5% of patients) were rituximab (22%), dexamethasone (13%), gemcitabine (12%), prednisone (11%), oxaliplatin (8%), etoposide (8%), and vincristine (6%).
Of 98 patients who were enrolled and underwent leukapheresis, 97 patients received infusion with KYMRIAH and one patient without measurable disease did not receive KYMRIAH. There were no manufacturing failures for the 98 enrolled patients. Of the 97 patients infused with KYMRIAH, the efficacy evaluable population, as specified in the protocol, included the first 90 patients with measurable disease who received KYMRIAH consecutively and had at least 9 months follow-up from first objective response or discontinued earlier.
Among the 90 patients with FL included in the efficacy analysis, the median age was 58 years (range: 29 to 73 years), 31% were female, 78% were White, 10% were Asian, and 1% were Black or African American. The median number of prior therapies was 4 (range: 2 to 13), with 24% receiving 2 prior lines, 21% receiving 3 prior lines, and 54% receiving ≥4 prior lines. Eighty-seven percent had Stage III-IV disease at study entry, 64% had bulky disease, 36% had a prior autologous HSCT, 79% were refractory to the most recent regimen, and 66% had progression within 24 months of initiating their first anti-CD20 combination therapy (POD24).
Efficacy was established on the basis of objective response rate and duration of response (DOR) as determined by an independent review committee (Table 14 and Table 15). The first disease assessment was scheduled to be performed at Month 3 post-infusion; the median time to first response was 2.9 months (range: 0.6 to 6.0 months). All responders achieved their response (complete response [CR] or partial response [PR]) at the first performed post-infusion disease assessment.
Table 14. Response Rates in Patients with Relapsed or Refractory FL aTwo patients, included in the Primary Efficacy Population, with best overall response of CR, had their disease relapsed more than 6 months after the last line of therapy.
bOf the 30 patients who initially achieved a PR, 14 patients (47%) converted to a CR, including 10 patients at the next subsequent visit and within 6 months post-infusion.Response Primary Efficacy Population
N = 90All Leukapheresed Patients
N = 98Overall response rate (ORR), n (%)
(95% CI)77 (86%)
(76.6, 92.1)84 (86%)
(77.2, 92.0)Complete response rate (CRR)a,b, n (%)
(95% CI)61 (68%)
(57.1, 77.2)66 (67%)
(57.1, 76.5)Table 15. Duration of Response in Patients with Relapsed or Refractory FL CR, complete response; DOR, duration of response; NE, not estimable.
*The first disease assessment was scheduled to be performed at Month 3 post-infusion. The median follow up is the time from first objective response to last disease assessment.
aAmong responders. DOR measured from date of first objective response to date of progression or death from relapse.
bKaplan-Meier estimate in months.
cA + sign indicates a censored value.From N = 90 Overall DOR, months N = 77 Median (95% CI)a,b NE (15.6, NE) Rangec (0.03+, 21.1+) Median Follow-up 9.1* % event-free probability At 9 months (95% CI) 75.2 (63.5, 83.6) At 12 months (95% CI) 70.8 (58.0, 80.3) DOR if Best Response is CR, months N = 61 Median (95% CI) a,b NE (15.6, NE) Rangec (2.7, 21.1+) % event-free probability At 9 months (95% CI) 87.7 (75.8, 93.9) At 12 months (95% CI) 85.2 (72.2, 92.4) -
15 REFERENCES
1. Porter DL, Hwang WT, Frey N, et al (2015). Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia (Table S4A). Sci. Transl Med; 7(303):303ra139. DOI: 10.1126/scitranslmed.aac5415.
2. Lee DW, Gardner R, Porter DL, et al (2014). Current concepts in the diagnosis and management of cytokine release syndrome. Blood; 124(2):188-95.
3. Lee DW, Santomasso BD, Locke FL, et al (2019) ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant; 25(4):625-38.
4. Santomosso BD, Nastoupil LJ, Adkins S, et al (2021) Management of Immune-Related Adverse Events in Patients Treated With Chimeric Antigen Receptor T-Cell Therapy: ASCO Guideline. J Clin Oncol; 39(35):3978-92.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
KYMRIAH is supplied as a frozen suspension of genetically modified autologous T cells in an infusion bag(s) labeled for the specific recipient. KYMRIAH is shipped directly to the cell lab associated with the infusion center in a liquid nitrogen Dewar. Product and patient-specific labels are located inside the Dewar.
Ped ALL: NDC 0078-0846-19
DLBCL and FL: NDC 0078-0958-19
- Confirm patient identity upon receipt.
- Store infusion bag(s) in a temperature-monitored system less than or equal to minus 120°C, e.g., in the vapor phase of liquid nitrogen.
- Use closed, break-proof, leak-proof containers when transporting infusion bags within the facility.
- Thaw KYMRIAH prior to infusion [see Dosage and Administration (2)].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Ensure that patients understand the risk of manufacturing failure. This has been reported in up to 9% of manufacturing attempts. In case of a manufacturing failure, a second manufacturing of KYMRIAH may be attempted. In addition, while the patient awaits the product, additional chemotherapy (not the lymphodepletion) may be necessary and may increase the risk of adverse events during the pre-infusion period.
Prior to infusion, advise patients of the following risks:
-
Cytokine Release Syndrome (CRS) -- Report signs and symptoms of CRS (high fever, difficulty breathing, chills/shaking chills, severe nausea, severe vomiting, severe diarrhea, severe muscle pain, severe joint pain, very low blood pressure, or dizziness/lightheadedness) to their healthcare professional [see Warnings and Precautions (5.1), Adverse Reactions (6.1)].
-
Neurological Toxicities -- Report altered or decreased consciousness, delirium, confusion, agitation, seizures, difficulty speaking and understanding, or loss of balance to their healthcare professional [see Warnings and Precautions (5.2), Adverse Reactions (6.1)].
- Hemophagocytic Lymphohistiocytosis (HLH)/Macrophage Activation Syndrome (MAS) -- Presenting signs and symptoms are similar to those of CRS and infections [see Warnings and Precautions (5.4), Adverse Reactions (6.1)].
-
Serious Infections -- KYMRIAH may cause serious infections. Advise patients that they will be screened for HBV, HCV, and HIV before collection of cells [see Warnings and Precautions (5.5), Adverse Reactions (6.1)].
-
Hypogammaglobulinemia -- Patients may need to receive immunoglobulin replacement for an indefinite amount of time following treatment with KYMRIAH. Patients should tell their physician about their treatment with KYMRIAH before receiving a live vaccine [see Warnings and Precautions (5.7), Adverse Reactions (6.1)].
- Driving and Engaging in Hazardous Occupations -- Patients should refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, for at least 8 weeks after treatment [see Warnings and Precautions (5.9)].
- Prolonged Cytopenia -- Patient may exhibit signs or symptoms associated with bone marrow suppression (i.e., neutropenia, thrombocytopenia and anemia) for several weeks following lymphodepleting chemotherapy and KYMRIAH.
Patients should be instructed to contact Novartis Pharmaceuticals Corporation at 1-844-4KYMRIAH if they get secondary malignancies [see Warnings and Precautions (5.8)].
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936
US License Number 1244© Novartis
T2022-31
-
Cytokine Release Syndrome (CRS) -- Report signs and symptoms of CRS (high fever, difficulty breathing, chills/shaking chills, severe nausea, severe vomiting, severe diarrhea, severe muscle pain, severe joint pain, very low blood pressure, or dizziness/lightheadedness) to their healthcare professional [see Warnings and Precautions (5.1), Adverse Reactions (6.1)].
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: May 2022 MEDICATION GUIDE
KYMRIAH® (pronounced KIM-RYE-AH)
(tisagenlecleucel)
Read this Medication Guide before you start your KYMRIAH treatment. The more you know about your treatment, the more active you can be in your care. Talk with your healthcare provider if you have questions about your health condition or treatment. Reading this Medication Guide does not take the place of talking with your healthcare provider about your treatment. What is the most important information I should know about KYMRIAH?
KYMRIAH may cause side effects that are severe or life-threatening. Call your healthcare provider or get emergency help right away if you get any of the following:- difficulty breathing
- fever (100.4°F/38°C or higher)
- chills/shaking chills
- confusion
- severe nausea, vomiting, diarrhea
- severe muscle or joint pain
- very low blood pressure
- dizziness/lightheadedness
It is important that you tell your healthcare providers that you have received KYMRIAH. Your healthcare providers may give you other medicines to treat your side effects.
What is KYMRIAH?
KYMRIAH is made from your own white blood cells and is a prescription cancer treatment used in patients up to 25 years old who have acute lymphoblastic leukemia (ALL) that is either relapsing (went into remission, then came back) or refractory (did not go into remission after receiving other leukemia treatments). It is also used in patients with large B-cell lymphoma or follicular lymphoma, two types of non-Hodgkin lymphoma, that have relapsed or are refractory after having at least two other kinds of treatment.
How will I get KYMRIAH?
Since KYMRIAH is made from your own white blood cells, your healthcare provider has to take some of your blood. This is called “leukapheresis.” It takes 3 to 6 hours and may need to be repeated. A tube (intravenous catheter) will be placed in your vein to collect your blood.
Your blood cells are frozen and sent to the manufacturing site to make KYMRIAH. It takes about 3-4 weeks from the time your cells are received at the manufacturing site and shipped back to your healthcare provider, but the time may vary.
While waiting for KYMRIAH to be made, your healthcare provider may give you therapy to stabilize your cancer.
In addition, before you get KYMRIAH, your healthcare provider may give you chemotherapy for a few days to prepare your body.
When your body is ready, your healthcare provider will give you KYMRIAH through a tube (intravenous catheter) in your vein. This usually takes less than one hour.
You should plan to stay within 2 hours of the location where you received your treatment for at least 4 weeks after getting KYMRIAH. Your healthcare provider will check to see if your treatment is working and help you with any side effects that occur.What should I avoid after receiving KYMRIAH? - Do not drive, operate heavy machinery, or do other dangerous things for 8 weeks after you get KYMRIAH because the treatment can cause temporary memory and coordination problems, including sleepiness, confusion, weakness, dizziness, and seizures.
- Do not donate blood, organs, tissues, sperm, oocytes, and other cells.
What are the possible or reasonably likely side effects of KYMRIAH?
The most common side effects of KYMRIAH are:
- difficulty breathing
- fever (100.4°F/38°C or higher)
- chills/shaking chills
- confusion
- severe nausea, vomiting, diarrhea
- severe muscle or joint pain
- very low blood pressure
- dizziness/lightheadedness
- headache
KYMRIAH can increase the risk of life-threatening infections that may lead to death. Tell your healthcare provider right away if you develop fever, chills, or any signs or symptoms of an infection.
KYMRIAH can lower one or more types of your blood cells (red blood cells, white blood cells, or platelets). After treatment, your healthcare provider will test your blood to check for this. Tell your healthcare provider right away if you get a fever, are feeling tired, weak, or short of breath, or have bruising or bleeding.
Having KYMRIAH in your blood may cause a false-positive HIV test result by some commercial tests.
These are not all the possible side effects of KYMRIAH. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of KYMRIAH.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
Do not use KYMRIAH for a condition for which it was not prescribed.
Talk to your healthcare provider about any concerns. You can ask your healthcare provider for information about KYMRIAH that is written for healthcare professionals.
For more information, go to KYMRIAH.com or call 1-844-NVS-CART (1-844-687-2278).
Manufactured and Distributed by: Novartis Pharmaceuticals Corporation, East Hanover, New Jersey 07936.
US License Number 1244
© NovartisT2022-32
- difficulty breathing
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KYMRIAH
tisagenlecleucel injection, suspensionProduct Information Product Type CELLULAR THERAPY Item Code (Source) NDC:0078-0846 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TISAGENLECLEUCEL (UNII: Q6C9WHR03O) (TISAGENLECLEUCEL - UNII:Q6C9WHR03O) TISAGENLECLEUCEL 2000000 Inactive Ingredients Ingredient Name Strength DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALBUMIN HUMAN (UNII: ZIF514RVZR) DEXTRAN 40 (UNII: K3R6ZDH4DU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0078-0846-19 1 in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125646 08/30/2017 KYMRIAH
tisagenlecleucel injection, suspensionProduct Information Product Type CELLULAR THERAPY Item Code (Source) NDC:0078-0958 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TISAGENLECLEUCEL (UNII: Q6C9WHR03O) (TISAGENLECLEUCEL - UNII:Q6C9WHR03O) TISAGENLECLEUCEL 60000000 Inactive Ingredients Ingredient Name Strength DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALBUMIN HUMAN (UNII: ZIF514RVZR) DEXTRAN 40 (UNII: K3R6ZDH4DU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0078-0958-19 1 in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125646 05/01/2018 Labeler - Novartis Pharmaceuticals Corporation (002147023)