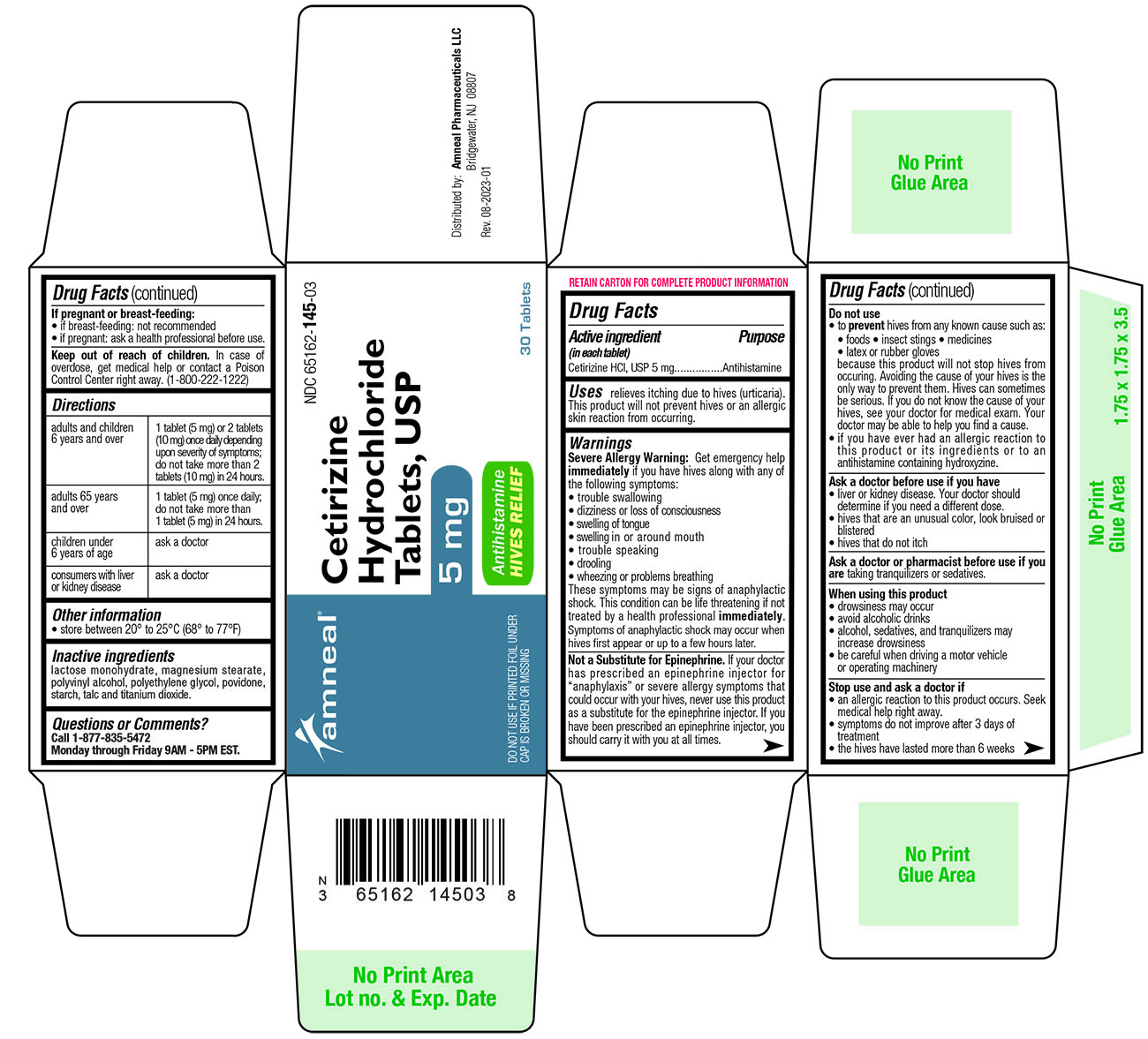
Label: CETIRIZINE HYDROCHLORIDE HIVES RELIEF- cetirizine tablet
- NDC Code(s): 65162-145-03, 65162-145-50
- Packager: Amneal Pharmaceuticals LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS AND USAGE
-
WARNINGS
Severe Allergy Warning: Get emergency help immediately if you have hives along
with any of the following symptoms:
• trouble swallowing
• dizziness or loss of consciousness
• swelling of tongue
• swelling in or around mouth
• trouble speaking
• drooling
• wheezing or problems breathing
These symptoms may be signs of anaphylactic shock. This condition can be life threatening if not treated by a health professional immediately. Symptoms of anaphylactic shock may occur when hives first appear or up to a few hours later.
Not a Substitute for Epinephrine . If your doctor has prescribed an epinephrine injector for “anaphylaxis” or severe allergy symptoms that could occur with your hives, never use this product as a substitute for the epinephrine injector. If you have been prescribed an epinephrine injector, you should carry it with you at all times.
-
DO NOT USE
Do not use
• to prevent hives from any known cause such as:
• foods • insect stings • medicines • latex or rubber gloves
because this product will not stop hives from occurring. Avoiding the cause of your hives is the only way to prevent them. Hives can sometimes be serious. If you do not know the cause of your hives, see your doctor for medical exam. Your doctor may be able to help you find a cause.
• if you have ever had an allergic reaction to this product or its ingredients or to an antihistamine containing hydroxyzine.
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
Adults and children 6years and over 1 tablet (5 mg) or 2 tablets (10 mg) once daily depending upon severity of symptoms; do not take more than 2 tablets (10 mg) in 24 hours. Adults 65years and over 1 tablet (5 mg) once daily; do not take more than 1 tablet (5 mg) in 24 hours. Children under 6 years of age ask a doctor Consumers with liver or kidney disease ask a doctor - OTHER INFORMATION
- INACTIVE INGREDIENTS
- OTC - QUESTIONS
- SPL UNCLASSIFIED SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CETIRIZINE HYDROCHLORIDE HIVES RELIEF
cetirizine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65162-145 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape OVAL Size 6mm Flavor Imprint Code IP;45 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65162-145-03 1 in 1 CARTON 01/21/2010 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:65162-145-50 500 in 1 BOTTLE; Type 0: Not a Combination Product 01/21/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078780 01/21/2010 Labeler - Amneal Pharmaceuticals LLC (123797875) Establishment Name Address ID/FEI Business Operations Amneal Pharmaceuticals of New York, LLC 831227801 analysis(65162-145) , label(65162-145) , manufacture(65162-145) , pack(65162-145)