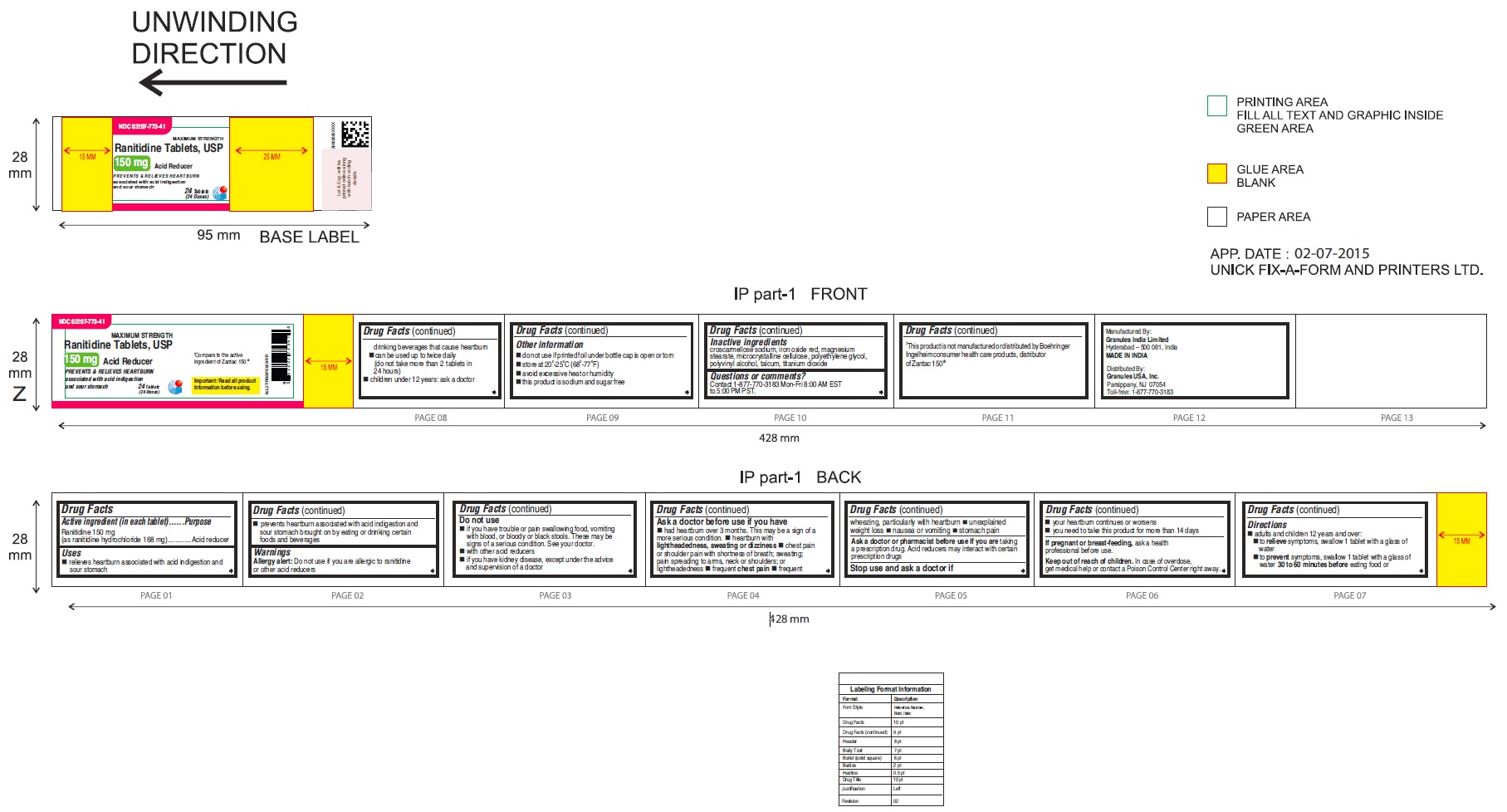
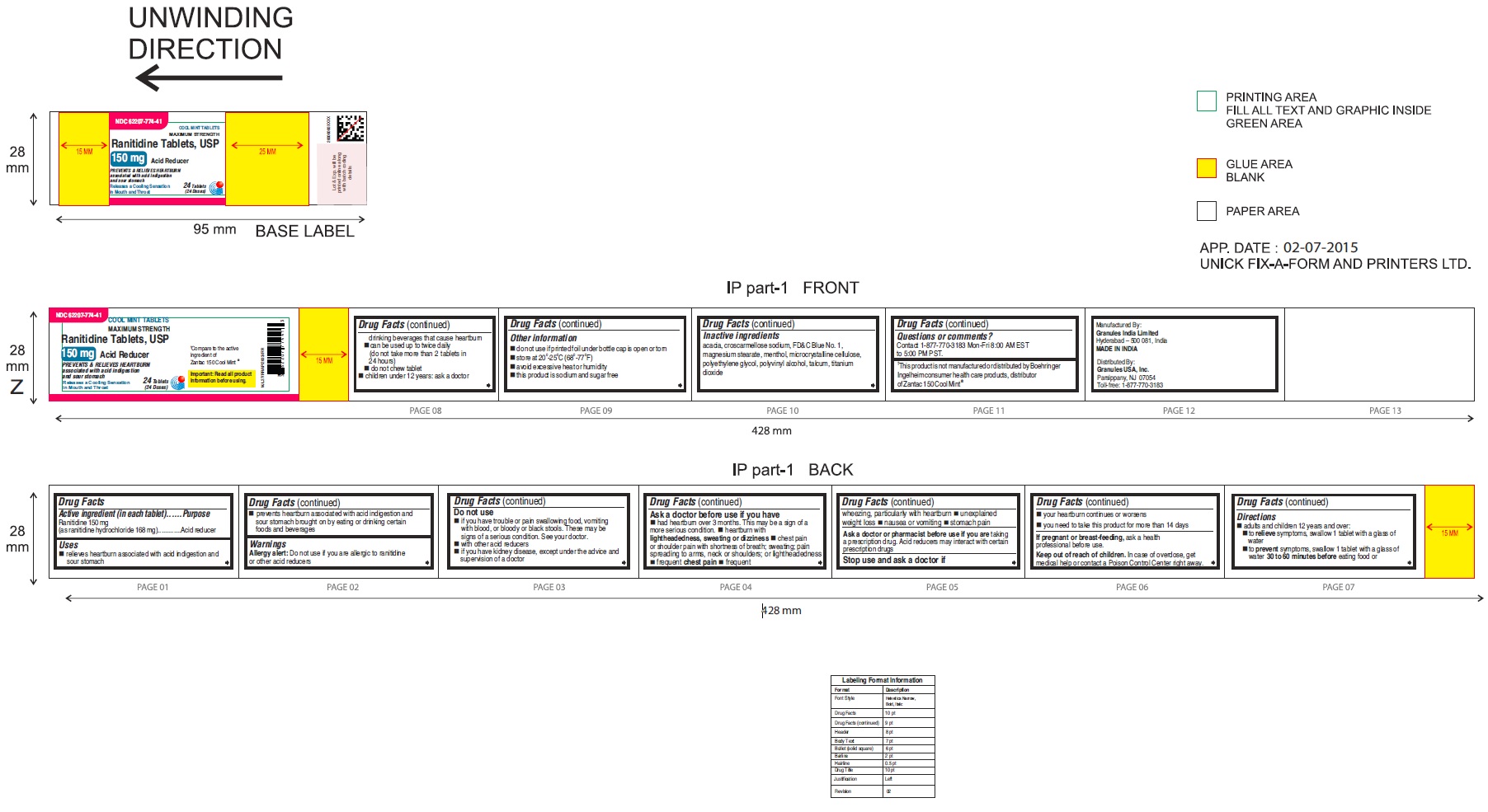
Label: RANITIDINE tablet
RANITIDINE COOL MINT- ranitidine tablet
-
NDC Code(s):
62207-773-32,
62207-773-36,
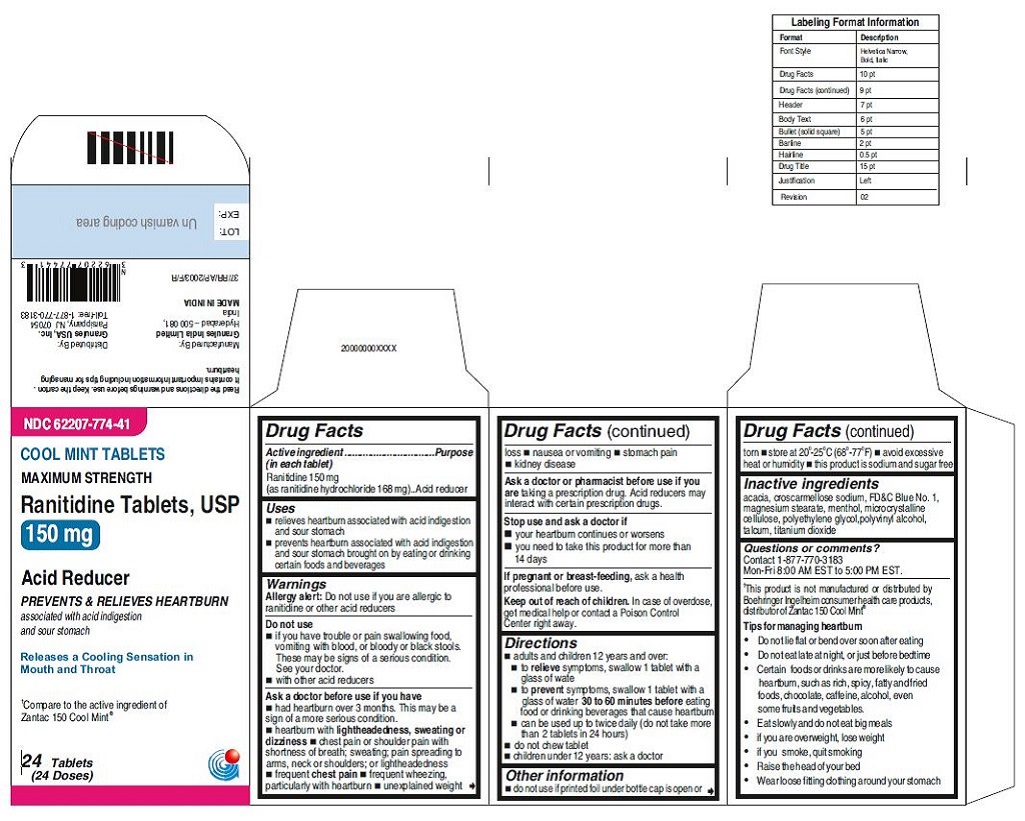
62207-773-41,
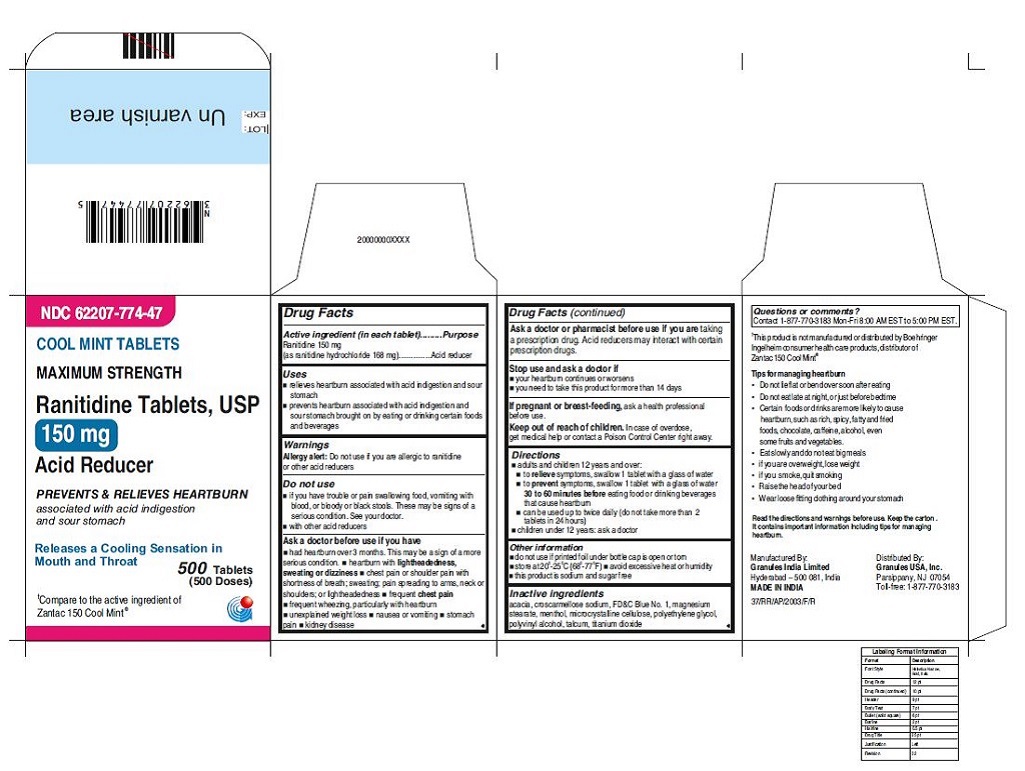
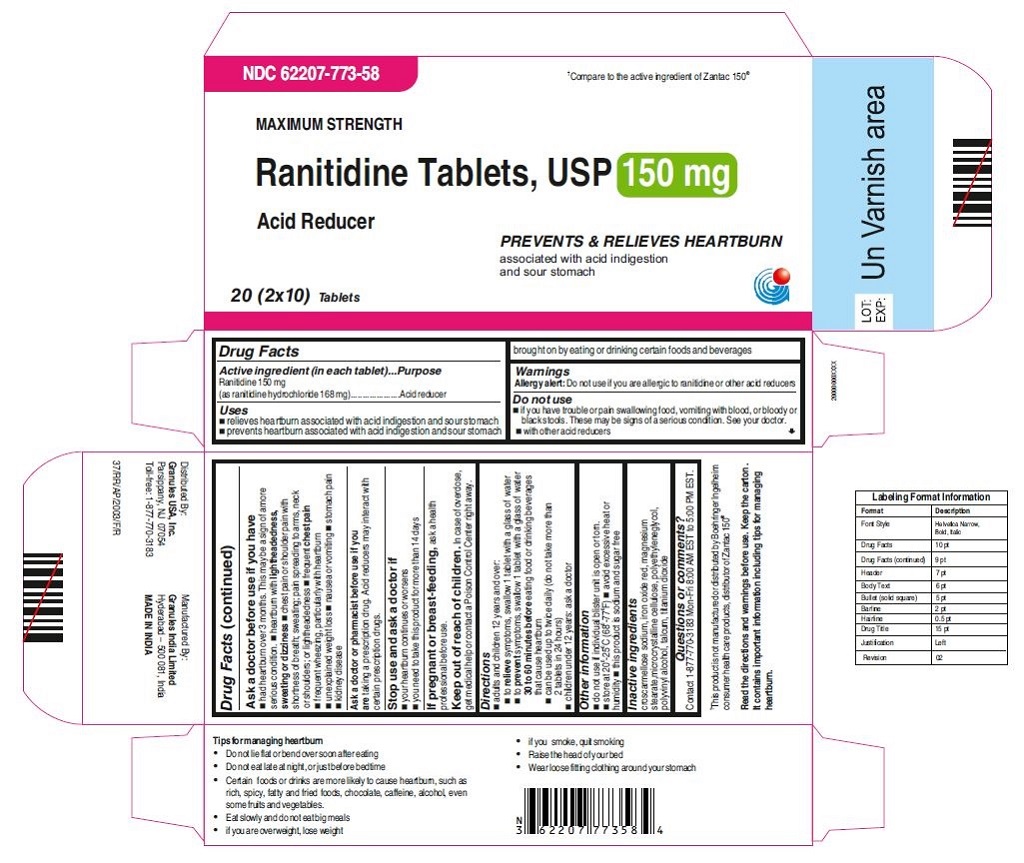
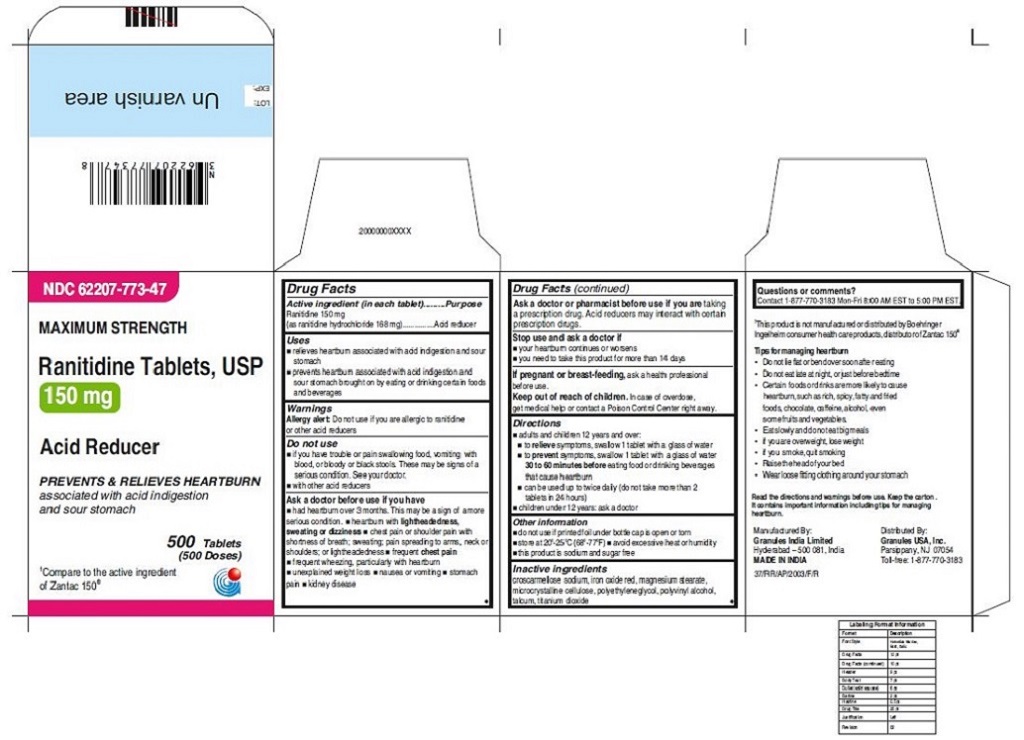
62207-773-47, view more62207-773-58, 62207-774-36, 62207-774-41, 62207-774-47, 62207-774-58
- Packager: Granules India Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
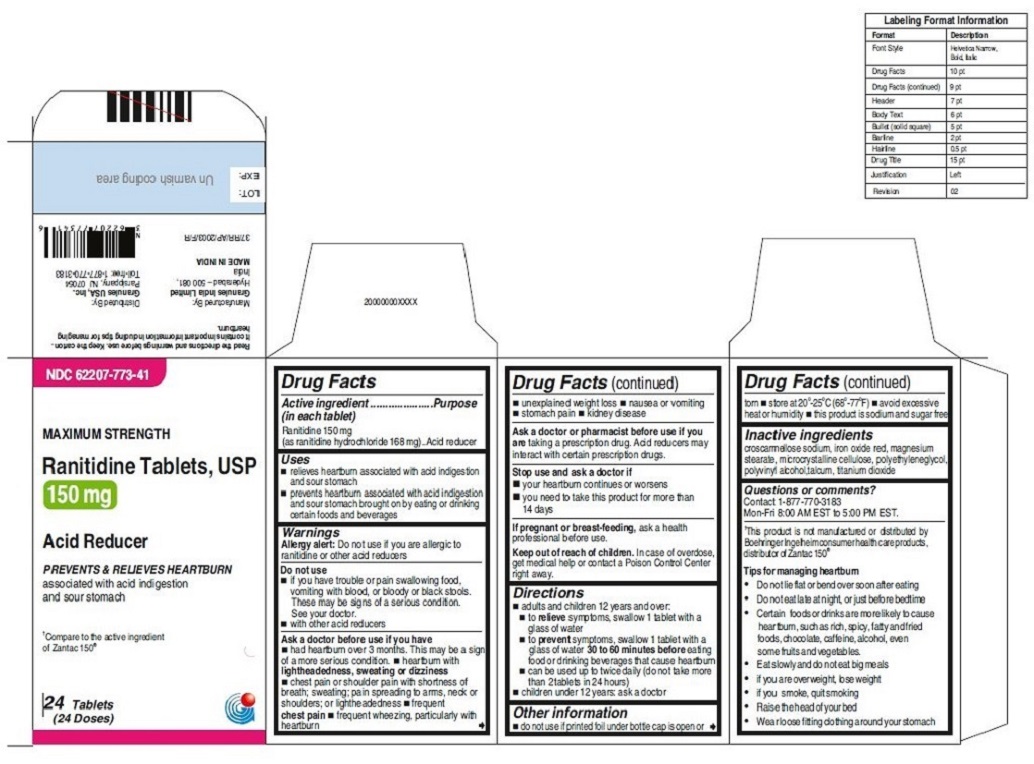
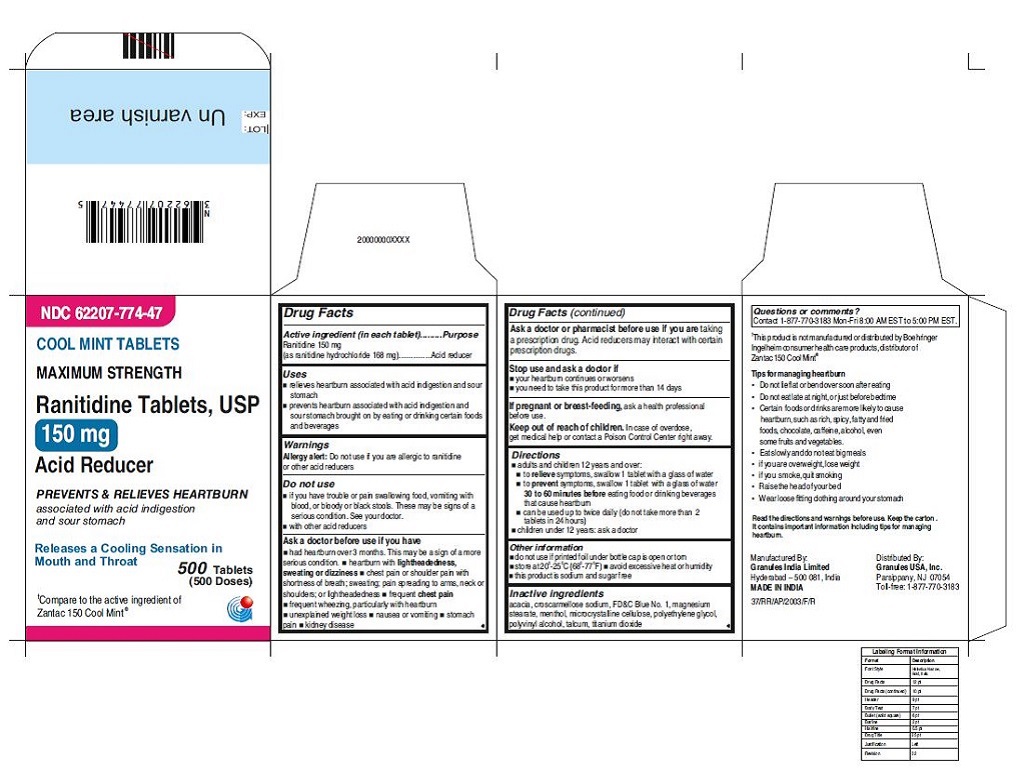
- ACTIVE INGREDIENT(S)
- PURPOSE
- USE(S)
- WARNINGS
- DO NOT USE
-
ASK A DOCTOR BEFORE USE IF
◾ had heartburn over 3 months. This may be a sign of a more serious condition.
◾ heartburn with lightheadedness, sweating or dizziness
◾ chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
◾ frequent chest pain
◾ frequent wheezing, particularly with heartburn
◾ unexplained weight loss
◾ nausea or vomiting
◾ stomach pain◾ kidney disease
Ask a doctor or pharmacist before use if you are taking a prescription drug. Acid reducers may interact with certain prescription drugs.
- STOP USE AND ASK DOCTOR IF
- PREGNANCY/BREASTFEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
◾ adults and children 12 years and over:
◾ to relieve symptoms, swallow 1 tablet with a glass of water
◾ to prevent symptoms, swallow 1 tablet with a glass of water ◾ 30 to 60 minutes before eating food or drinking beverages that cause heartburn
◾ can be used up to twice daily (do not take more than 2 tablets in 24 hours)
◾ do not chew tablet (for cool mint tablets only)
◾ children under 12 years: ask a doctor - OTHER INFORMATION
-
INACTIVE INGREDIENTS
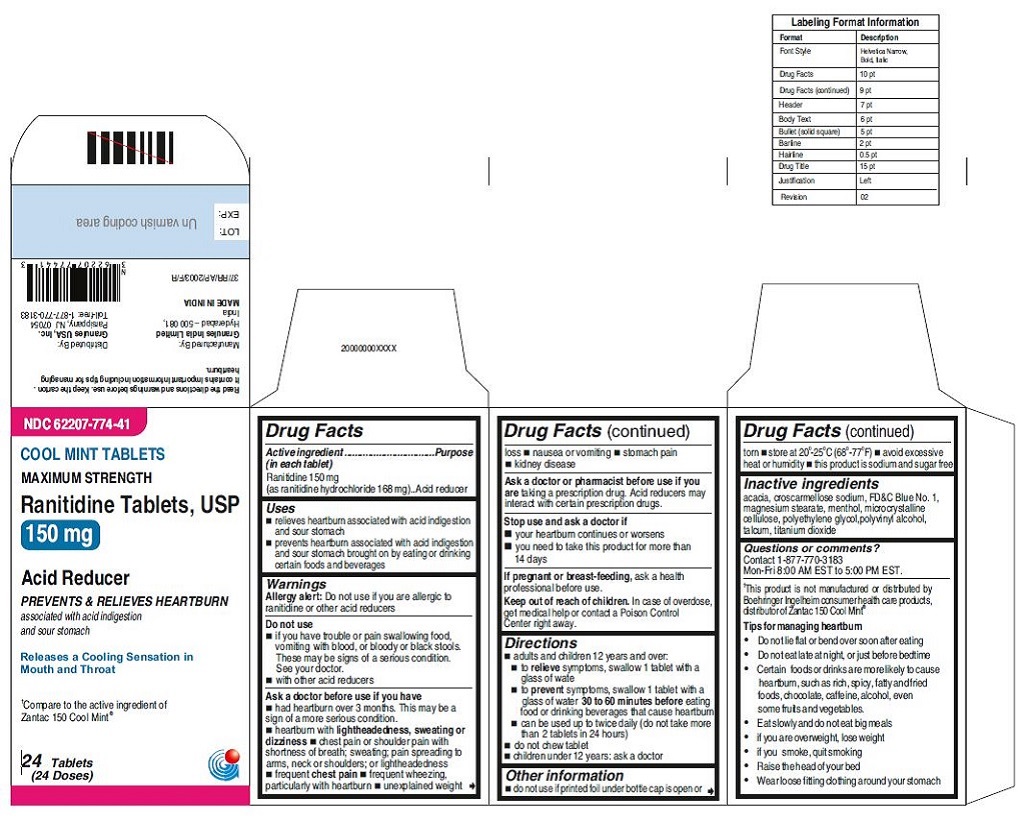
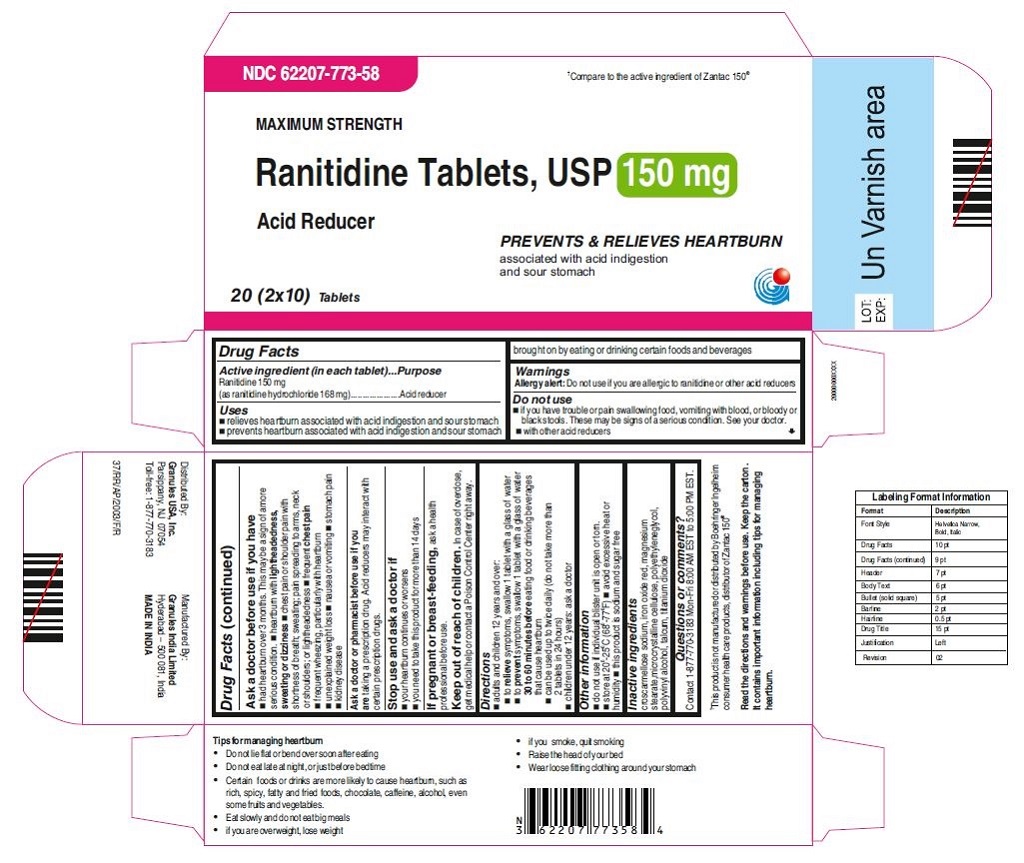
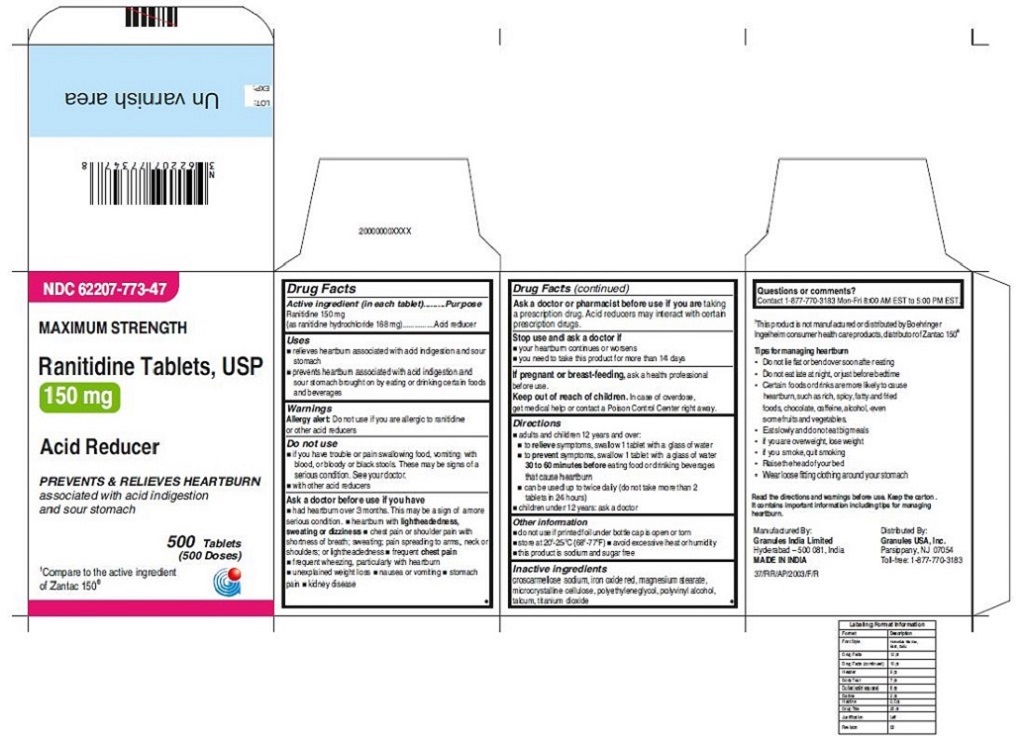
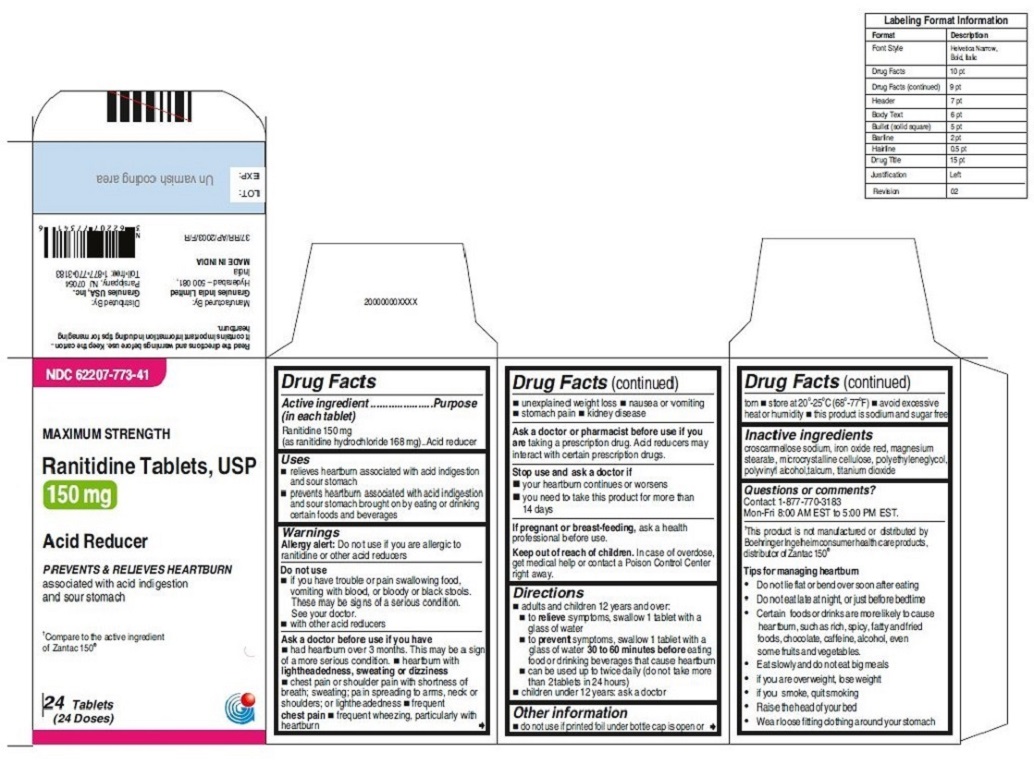
For 150 mg:croscarmellose sodium, iron oxide red, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol,talcum, titanium dioxide
For 150 mg cool mint:acacia, croscarmellose sodium, FD&C Blue No. 1,magnesium stearate, menthol, microcrystalline cellulose,polyethylene glycol, polyvinyl alcohol, talcum,
titanium dioxide - Questions or comments?
-
Read the directions and warnings before use.
Keep the carton. It contains important information including tips for managing heartburn.
Tips for managing heartburn
•Do not lie flat or bend over soon after eating
•Do not eat late at night, or just before bedtime
•Certain foods or drinks are more likely to cause heartburn, such as rich, spicy, fatty andfried foods, chocolate, caffeine, alcohol, even some fruits and vegetables.
•Eat slowly and do not eat big meals
•if you are overweight, lose weight
•if you smoke, quit smoking
•Raise the head of your bed
•Wear loose fitting clothing around your stomach - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RANITIDINE
ranitidine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62207-773 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RANITIDINE HYDROCHLORIDE (UNII: BK76465IHM) (RANITIDINE - UNII:884KT10YB7) RANITIDINE 150 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FERRIC OXIDE RED (UNII: 1K09F3G675) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color brown Score no score Shape ROUND Size 9mm Flavor Imprint Code 150;G Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62207-773-58 2 in 1 CARTON 06/26/2019 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:62207-773-41 1 in 1 CARTON 06/26/2019 2 24 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:62207-773-47 1 in 1 CARTON 06/26/2019 3 500 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:62207-773-36 5 in 1 CARTON 06/26/2019 4 NDC:62207-773-32 10000 in 1 BAG; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210243 06/26/2019 RANITIDINE COOL MINT
ranitidine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62207-774 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RANITIDINE HYDROCHLORIDE (UNII: BK76465IHM) (RANITIDINE - UNII:884KT10YB7) RANITIDINE 150 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) MAGNESIUM STEARATE (UNII: 70097M6I30) MENTHOL (UNII: L7T10EIP3A) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color blue Score no score Shape ROUND Size 9mm Flavor MINT Imprint Code 150;G Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62207-774-58 2 in 1 CARTON 06/26/2019 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:62207-774-41 1 in 1 CARTON 06/26/2019 2 24 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:62207-774-47 1 in 1 CARTON 06/26/2019 3 500 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:62207-774-36 5 in 1 CARTON 06/26/2019 4 10000 in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210243 06/26/2019 Labeler - Granules India Ltd (915000087)