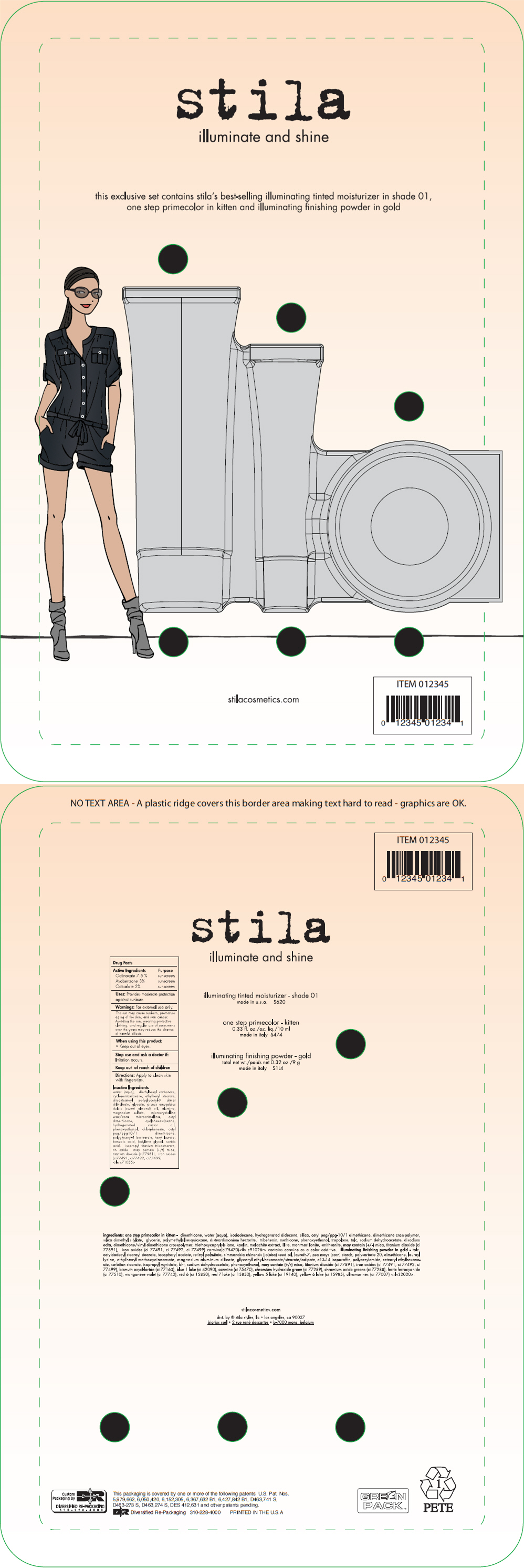
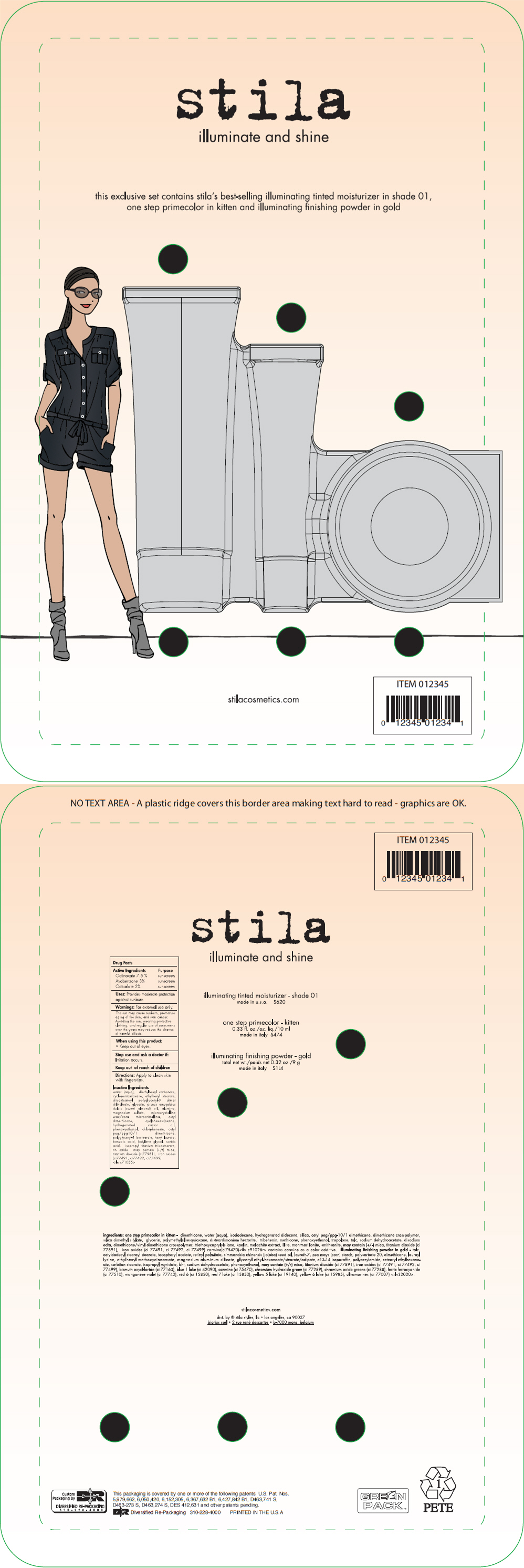
Label: STILA ILLUMINATE AND SHINE- octinoxate, avobenzoate, and octisalate kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 76049-620-01 - Packager: Stila Styles, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 25, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
-
Inactive Ingredients
water (aqua), diethylhexyl carbonate, cyclopentasiloxane, ethylhexyl stearate, diisostearoyl polyglyceryl-3 dimer dilinoleate, glycerin, prunus amygdalus dulcis (sweet almond) oil, alumina, magnesium sulfate, microcrystalline wax/cera microcristallina, cetyl dimethicone, cyclohexasiloxane, hydrogenated castor oil, phenoxyethanol, chlorphenesin, cetyl peg/ppg10/1 dimethicone, polyglyceryl-4 isostearate, hexyl laurate, benzoic acid, butylene glycol, sorbic acid, isopropyl titanium triisostearate, tin oxide may contain (+/-) mica, titanium dioxide (ci77981), iron oxides (ci77491, ci77492, ci77499) <iln c71055>
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Kit Label
-
INGREDIENTS AND APPEARANCE
STILA ILLUMINATE AND SHINE
octinoxate, avobenzoate, and octisalate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76049-620 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76049-620-01 1 in 1 PACKAGE, COMBINATION Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 50 mL Part 2 1 TUBE 10 mL Part 3 1 CONTAINER 9 g Part 1 of 3 ILLUMINATED TINTED MOISTURIZER SHADE 01
octinoxate, avobenzone, and octisalate lotionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 75 mg in 1 mL Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) diethylhexyl carbonate (UNII: YCD50O0Z6L) cyclomethicone 5 (UNII: 0THT5PCI0R) ethylhexyl stearate (UNII: EG3PA2K3K5) diisostearoyl polyglyceryl-3 dimer dilinoleate (UNII: G3232Z5S2O) glycerin (UNII: PDC6A3C0OX) almond oil (UNII: 66YXD4DKO9) aluminum oxide (UNII: LMI26O6933) magnesium sulfate (UNII: DE08037SAB) microcrystalline wax (UNII: XOF597Q3KY) cyclomethicone 6 (UNII: XHK3U310BA) hydrogenated castor oil (UNII: ZF94AP8MEY) phenoxyethanol (UNII: HIE492ZZ3T) chlorphenesin (UNII: I670DAL4SZ) polyglyceryl-4 isostearate (UNII: 820DPX33S7) hexyl laurate (UNII: 4CG9F9W01Q) benzoic acid (UNII: 8SKN0B0MIM) butylene glycol (UNII: 3XUS85K0RA) sorbic acid (UNII: X045WJ989B) isopropyl titanium triisostearate (UNII: 949E3KBJ1I) stannic oxide (UNII: KM7N50LOS6) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) ferrosoferric oxide (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 50 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/31/2013 Part 2 of 3 ONE STEP PRIMECOLOR KITTEN
foundations lotionProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR dimethicone (UNII: 92RU3N3Y1O) INGR water (UNII: 059QF0KO0R) INGR isododecane (UNII: A8289P68Y2) INGR hydrogenated didecene (UNII: 048B98MT5O) INGR silicon dioxide (UNII: ETJ7Z6XBU4) INGR silica dimethyl silylate (UNII: EU2PSP0G0W) INGR glycerin (UNII: PDC6A3C0OX) INGR disteardimonium hectorite (UNII: X687XDK09L) INGR tribehenin (UNII: 8OC9U7TQZ0) INGR phenoxyethanol (UNII: HIE492ZZ3T) INGR tropolone (UNII: 7L6DL16P1T) INGR talc (UNII: 7SEV7J4R1U) INGR sodium dehydroacetate (UNII: 8W46YN971G) INGR edetate disodium (UNII: 7FLD91C86K) INGR triethoxycaprylylsilane (UNII: LDC331P08E) INGR kaolin (UNII: 24H4NWX5CO) INGR montmorillonite (UNII: A585MN1H2L) INGR mica (UNII: V8A1AW0880) INGR titanium dioxide (UNII: 15FIX9V2JP) INGR ferric oxide red (UNII: 1K09F3G675) INGR ferric oxide yellow (UNII: EX438O2MRT) INGR ferrosoferric oxide (UNII: XM0M87F357) INGR carminic acid (UNII: CID8Z8N95N) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 10 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC Part 3 of 3 ILLUMINATING FINISHING POWDER GOLD
face powders powderProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR talc (UNII: 7SEV7J4R1U) INGR octyldodecyl stearoyl stearate (UNII: 3D47Q6D93C) INGR .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) INGR vitamin a palmitate (UNII: 1D1K0N0VVC) INGR jojoba oil (UNII: 724GKU717M) INGR laureth-7 (UNII: Z95S6G8201) INGR starch, corn (UNII: O8232NY3SJ) INGR polysorbate 20 (UNII: 7T1F30V5YH) INGR dimethicone (UNII: 92RU3N3Y1O) INGR lauroyl lysine (UNII: 113171Q70B) INGR octinoxate (UNII: 4Y5P7MUD51) INGR magnesium aluminum silicate (UNII: 6M3P64V0NC) INGR c13-14 isoparaffin (UNII: E4F12ROE70) INGR cetearyl ethylhexanoate (UNII: 9M64UO4C25) INGR sorbitan monostearate (UNII: NVZ4I0H58X) INGR isopropyl myristate (UNII: 0RE8K4LNJS) INGR butylated hydroxytoluene (UNII: 1P9D0Z171K) INGR sodium dehydroacetate (UNII: 8W46YN971G) INGR phenoxyethanol (UNII: HIE492ZZ3T) INGR mica (UNII: V8A1AW0880) INGR titanium dioxide (UNII: 15FIX9V2JP) INGR ferric oxide red (UNII: 1K09F3G675) INGR ferric oxide yellow (UNII: EX438O2MRT) INGR ferrosoferric oxide (UNII: XM0M87F357) INGR bismuth oxychloride (UNII: 4ZR792I587) INGR fd&c blue no. 1 (UNII: H3R47K3TBD) INGR carminic acid (UNII: CID8Z8N95N) INGR chromium hydroxide green (UNII: RV8FT8XF5R) INGR chromic oxide (UNII: X5Z09SU859) INGR ferric ferrocyanide (UNII: TLE294X33A) INGR manganese violet (UNII: 72M48QQV8Q) INGR d&c red no. 6 (UNII: 481744AI4O) INGR d&c red no. 7 (UNII: ECW0LZ41X8) INGR fd&c yellow no. 5 (UNII: I753WB2F1M) INGR fd&c yellow no. 6 (UNII: H77VEI93A8) INGR ultramarine blue (UNII: I39WR998BI) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 9 g in 1 CONTAINER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 08/31/2013 Labeler - Stila Styles, LLC (809192896) Establishment Name Address ID/FEI Business Operations GORDON LABORATORIES, INC. 008328619 MANUFACTURE(76049-620)