Label: PALL STERILE CORD BLOOD COLLECTION UNIT- sterile cord blood collection unit solution
- NDC Code(s): 79403-791-01
- Packager: Global Life Sciences Solutions USA LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ANTICOAGULANT CITRATE PHOSPHATE DEXTROSE SOLUTION (CPD)
-
Indications and Usage Section
For collection of up to 210 ml of umbilical cord blood. Use aseptic technique.
Contents inside overwrap pouch, within the foil envelope, are sterile and acceptable for use in a sterile field if pouch is unopened and undamaged; visual inspection to confirm the integrity of overwrap pouch should be performed.
- Warnings Section
- GENERAL PRECAUTIONS
- HOW SUPPLIED
-
INFORMATION FOR PATIENTS
Visit us at www.pall.com/medical
For Pall customer service, call: 1.800.645.6578
DonorCare is a registered trademark of ITL Corporation, Canberra, Australia. Produced under license from ThermoGenesis Corp
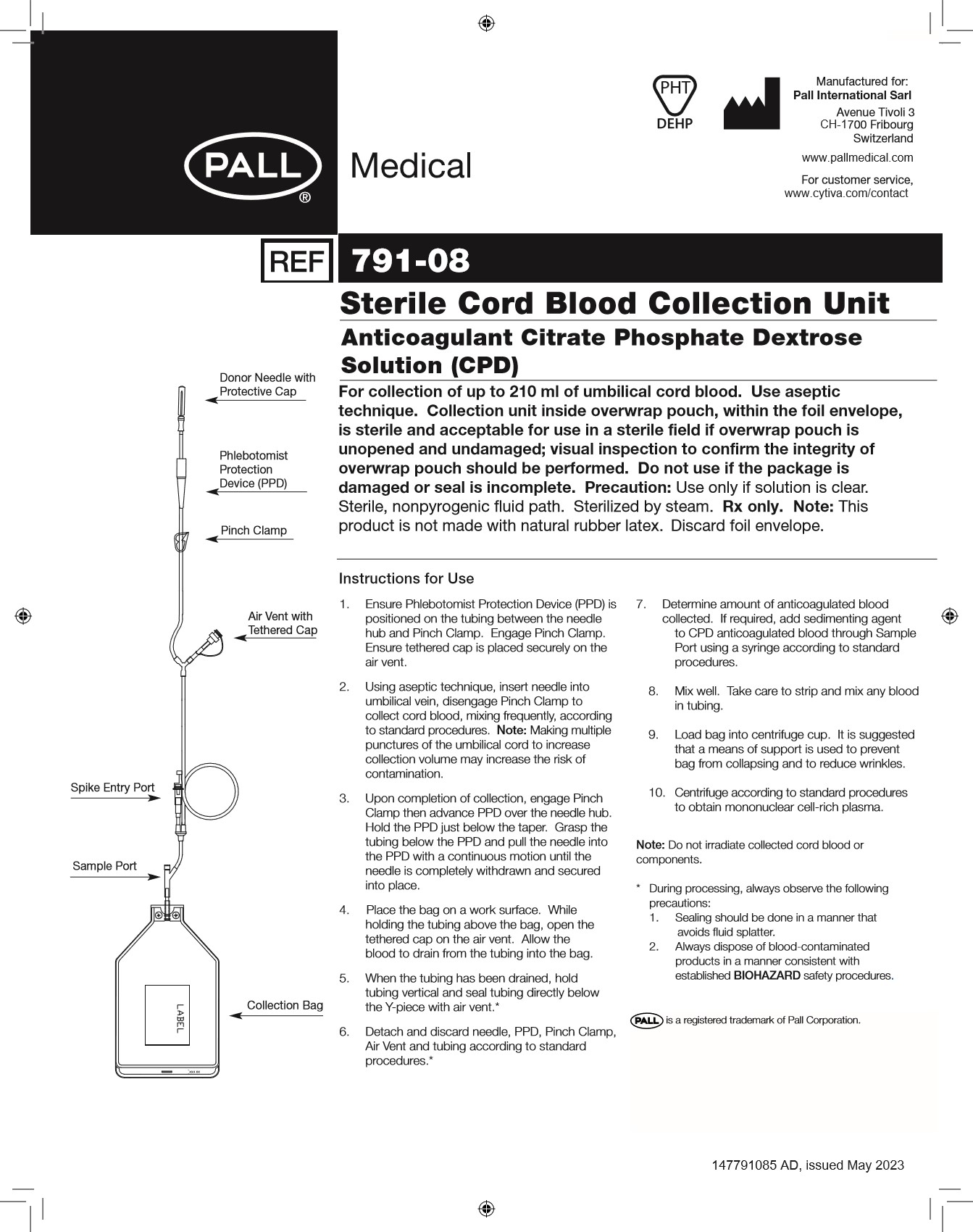
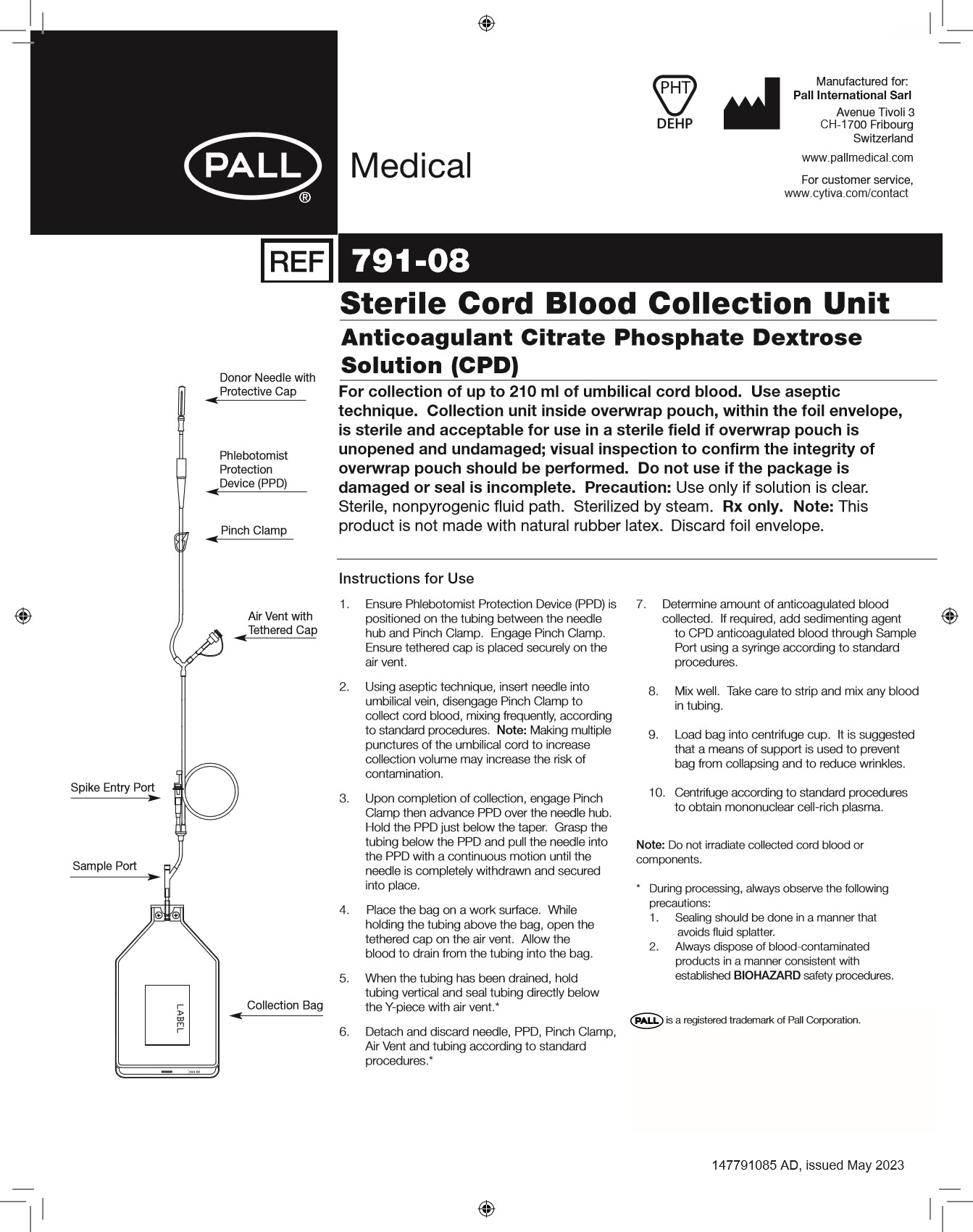
- Ensure DonorCare® Needle Guard (DCNG) is positioned on the tubing between the needle hub and Pinch Clamp. Engage Pinch Clamp. Ensure tethered cap is placed securely on the air vent.
- Using aseptic technique, insert needle into umbilical vein, disengage Pinch Clamp to collect cord blood, mixing frequently, according to standard procedures.
- Upon completion of collection, engage Pinch Clamp then withdraw needle from umbilical vein. Slide the DCNG midway over the needle hub. While holding the sides of DCNG near front, grasp tubing and pull smoothly, pulling needle into the DCNG until it locks into place. Confirm that needle is locked by listening for the second click as the needle is drawn into the DCNG. Ensure that tubing cannot be pulled through DCNG.
- Place the bag on a work surface. While holding the tubing above the bag, open the tethered cap on the air vent. Allow the blood to drain from the tubing into the bag.
- When the tubing has been drained, hold tubing vertical and seal tubing directly below the Y-piece with air vent.
- Detach and discard needle, DCNG, Pinch Clamp, Air Vent and tubing according to standard procedures.
- Determine amount of anticoagulated blood collected. If required, add sedimenting agent to CPD anticoagulated blood through Sample Port using a syringe according to standard procedures.
- Mix well. Take care to strip and mix any blood in tubing.
- Load bag into centrifuge cup. It is suggested that a means of support is used to prevent bag from collapsing and to reduce wrinkles.
- Centrifuge according to standard procedures to obtain mononuclear cell-rich plasma.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PALL STERILE CORD BLOOD COLLECTION UNIT
sterile cord blood collection unit solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:79403-791 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 0.893 g in 35 mL SODIUM CITRATE (UNII: 1Q73Q2JULR) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) SODIUM CITRATE 0.921 g in 35 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 0.114 g in 35 mL SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE 0.0078 g in 35 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 35 g in 35 mL PHOSPHORIC ACID (UNII: E4GA8884NN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79403-791-01 35 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN800222 03/16/2011 Labeler - Global Life Sciences Solutions USA LLC (011658242) Establishment Name Address ID/FEI Business Operations Haemonetics Manufacturing Inc. 078598396 manufacture(79403-791)