Label: BLAIR AND JACK ANTI-BUMP ACNE TREATMENT- benzoyl peroxide cream
- NDC Code(s): 84377-489-00
- Packager: Blair + Jack
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
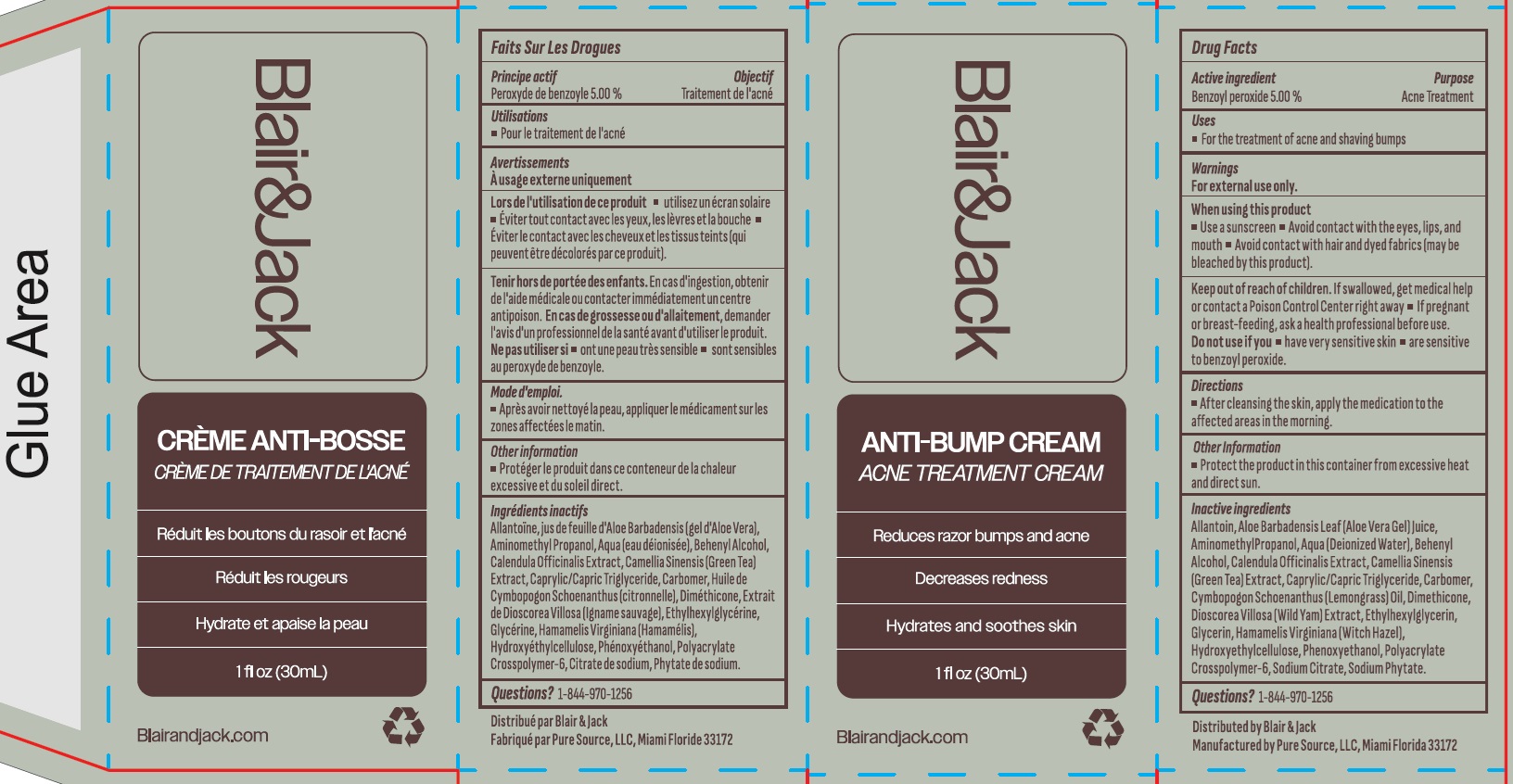
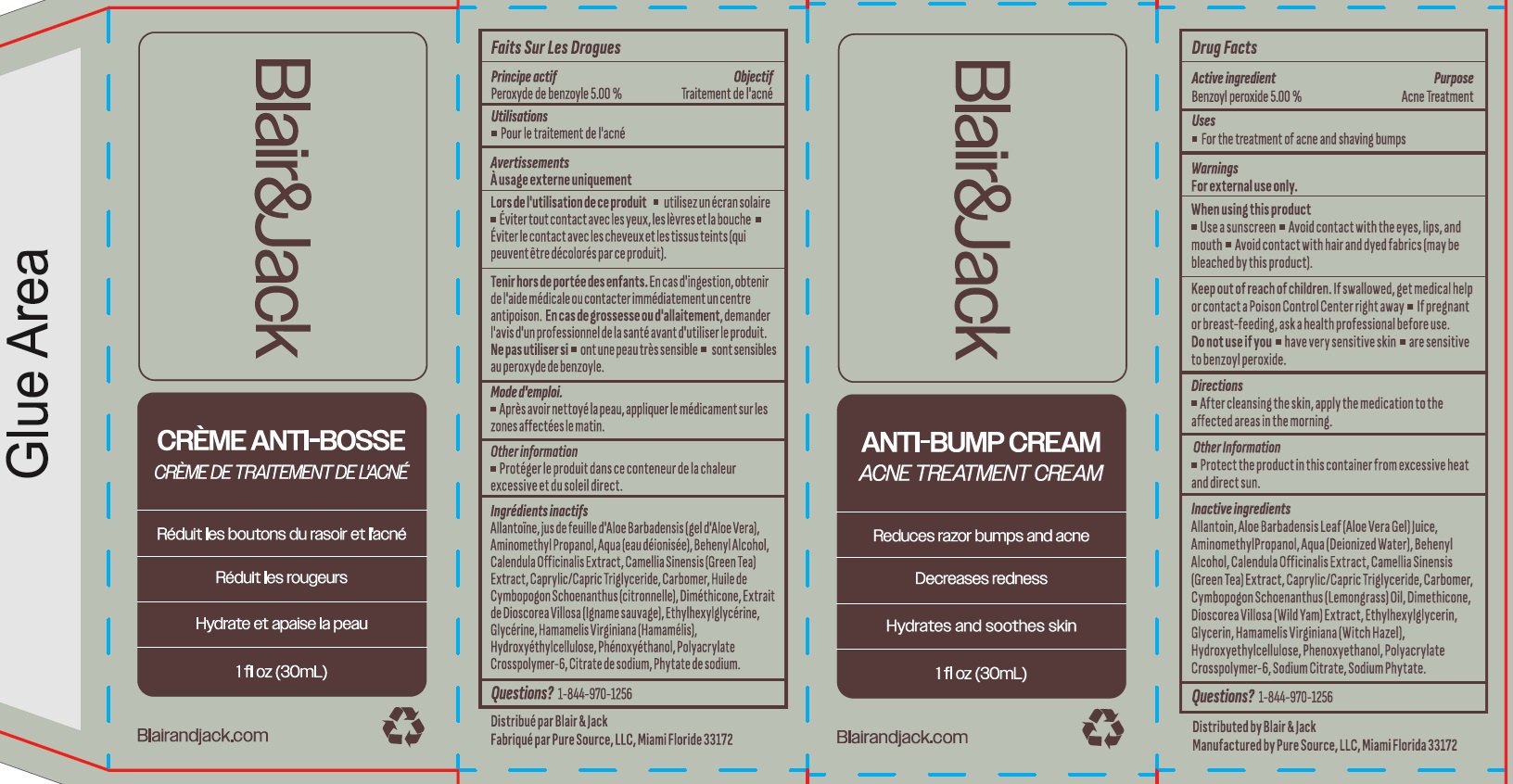
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Allantoin, Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aminomethyl Propanol, Aqua (Deionized Water), Behenyl Alcohol, Calendula Officinalis Extract, Camellia Sinensis (Green Tea) Extract, Caprylic/Capric Triglyceride, Carbomer, Cymbopogon Schoenanthus (Lemongrass) Oil, Dimethicone, Dioscorea Villosa (Wild Yam) Extract, Ethylhexylglycerin, Glycerin, Hamamelis Virginiana (Witch Hazel), Hydroxyethylcellulose, Phenoxyethanol, Polyacrylate Crosspolymer-6, Sodium Citrate, Sodium Phytate.
- Question?
- Package Labeling
-
INGREDIENTS AND APPEARANCE
BLAIR AND JACK ANTI-BUMP ACNE TREATMENT
benzoyl peroxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84377-489 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) WATER (UNII: 059QF0KO0R) DOCOSANOL (UNII: 9G1OE216XY) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) GREEN TEA LEAF (UNII: W2ZU1RY8B0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) CYMBOPOGON SCHOENANTHUS OIL (UNII: XE7K568ILO) DIMETHICONE (UNII: 92RU3N3Y1O) DIOSCOREA VILLOSA TUBER (UNII: IWY3IWX2G8) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) PHENOXYETHANOL (UNII: HIE492ZZ3T) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) PHYTATE SODIUM (UNII: 88496G1ERL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84377-489-00 1 in 1 BOX 06/17/2024 1 30 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/17/2024 Labeler - Blair + Jack (209190901)