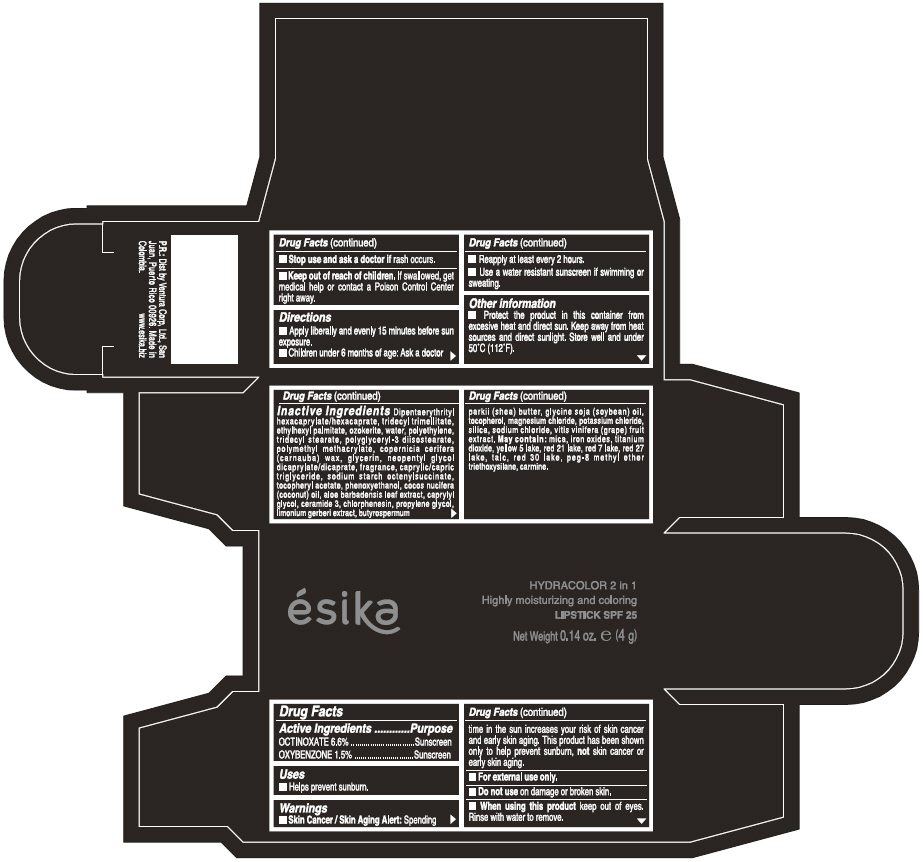
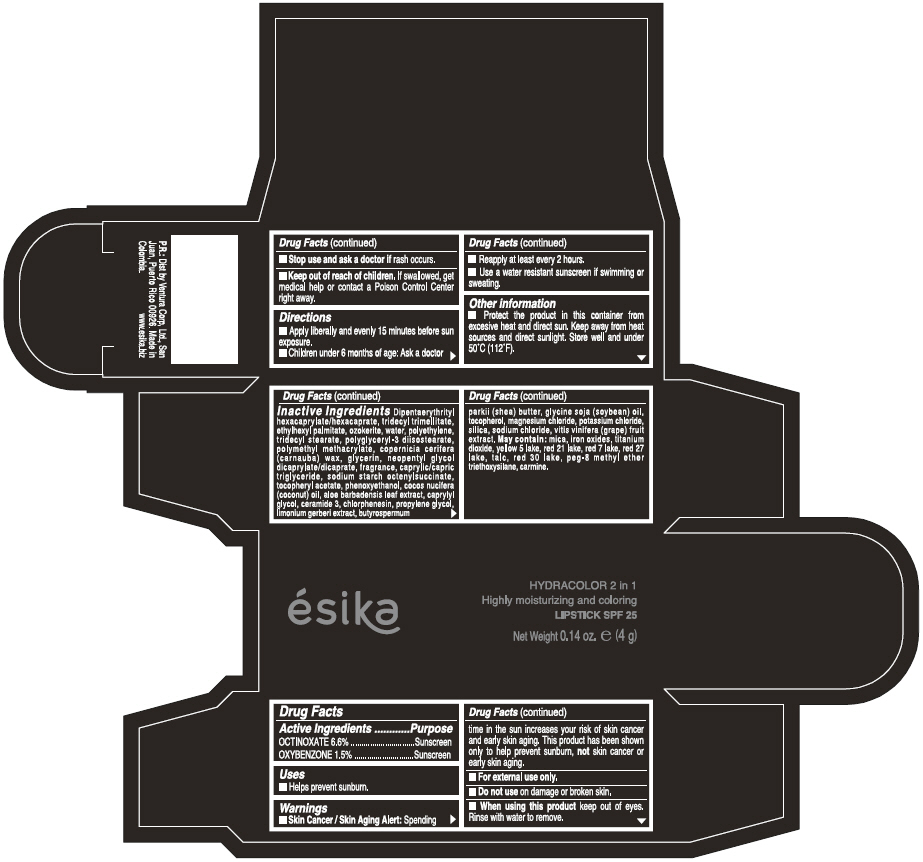
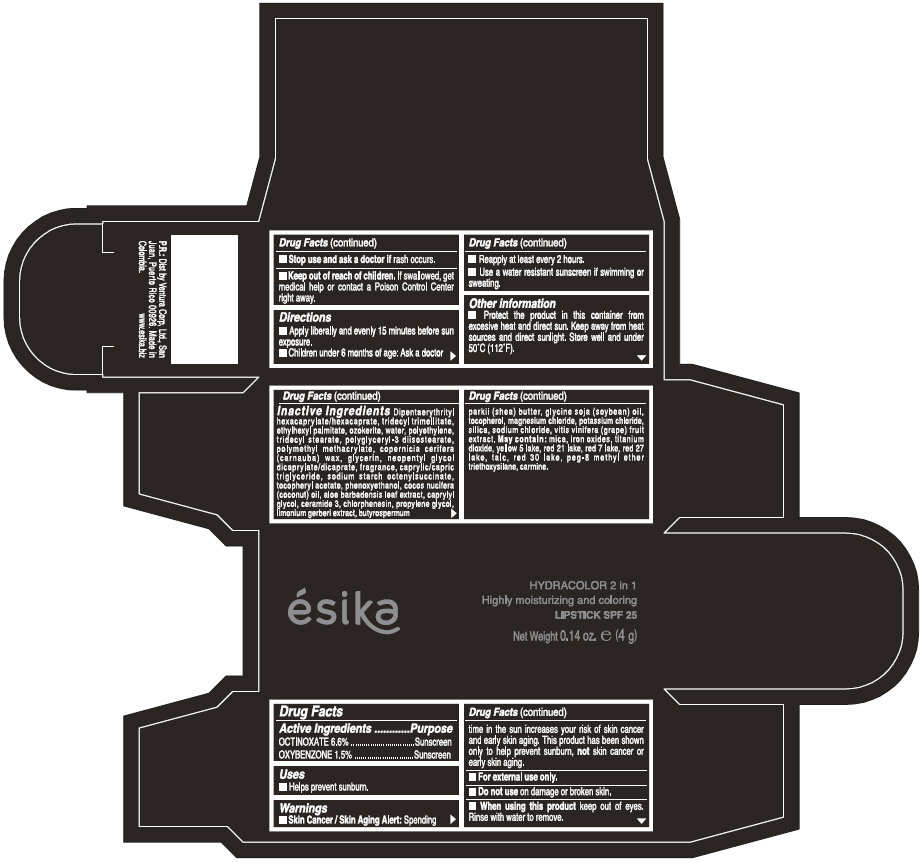
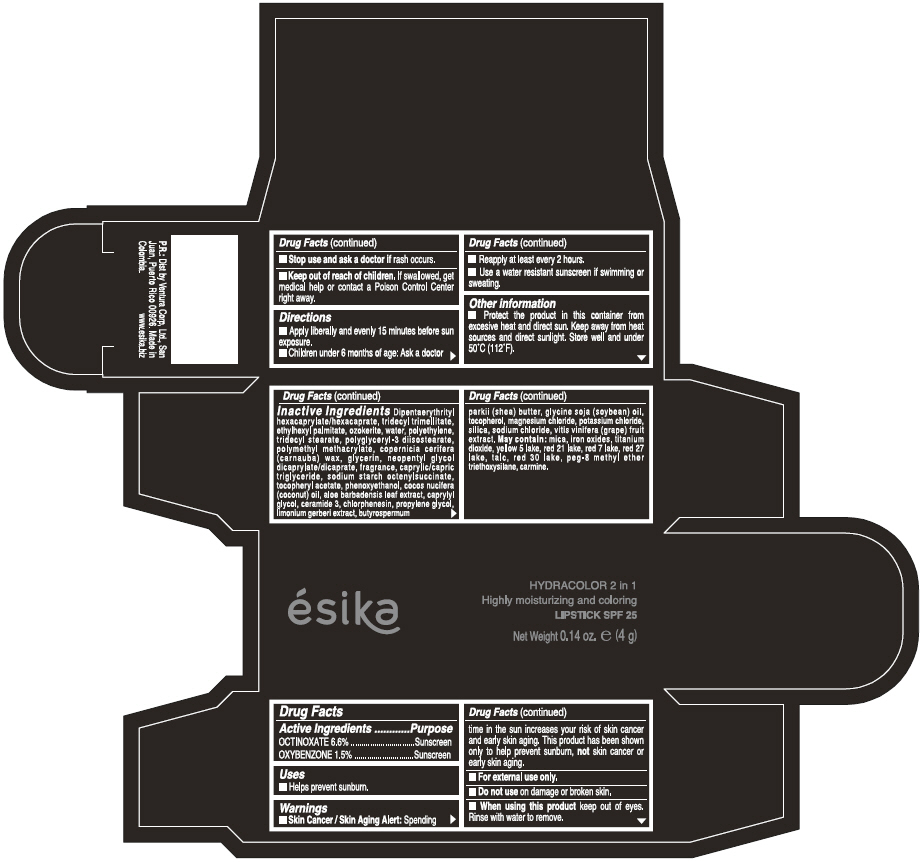
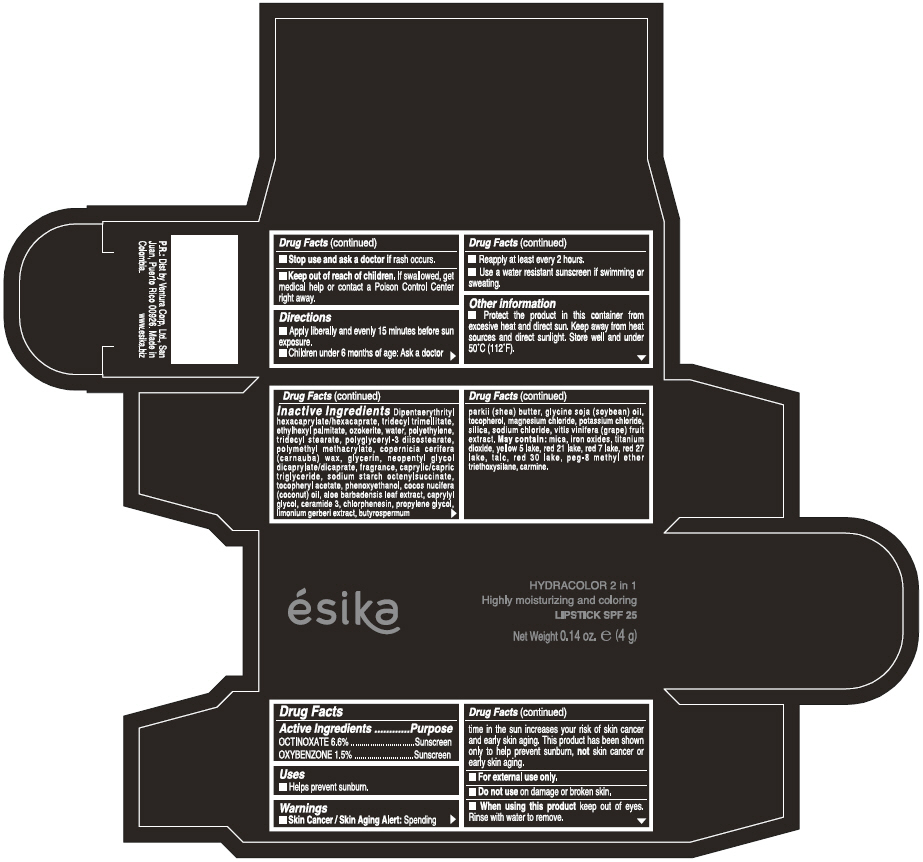
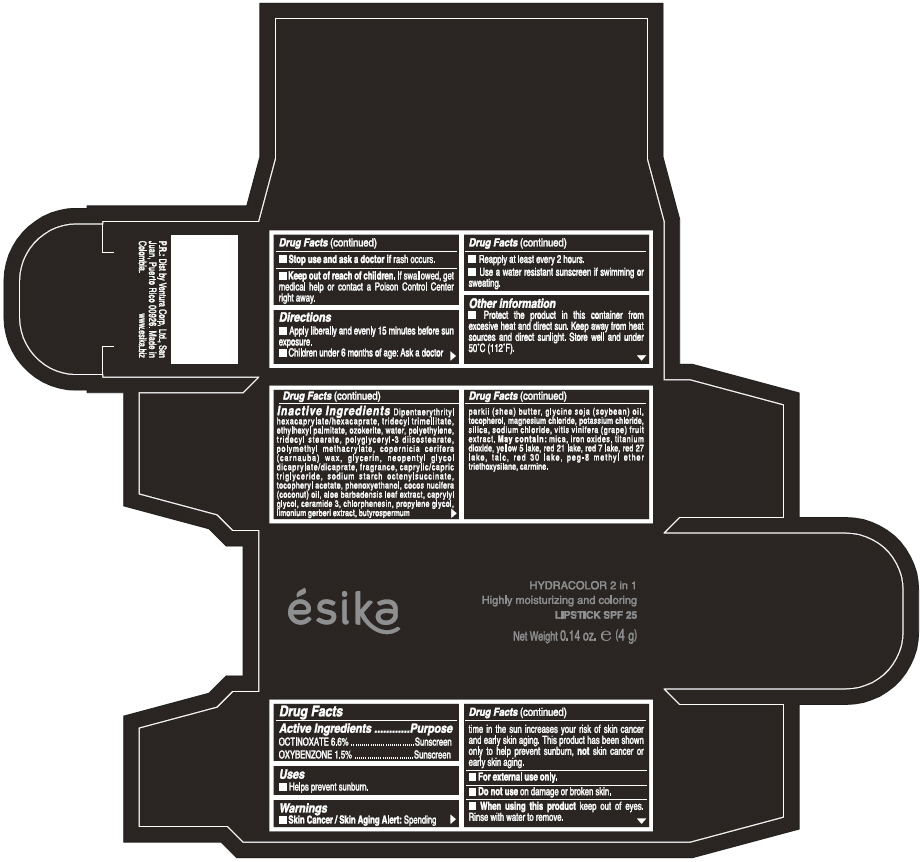
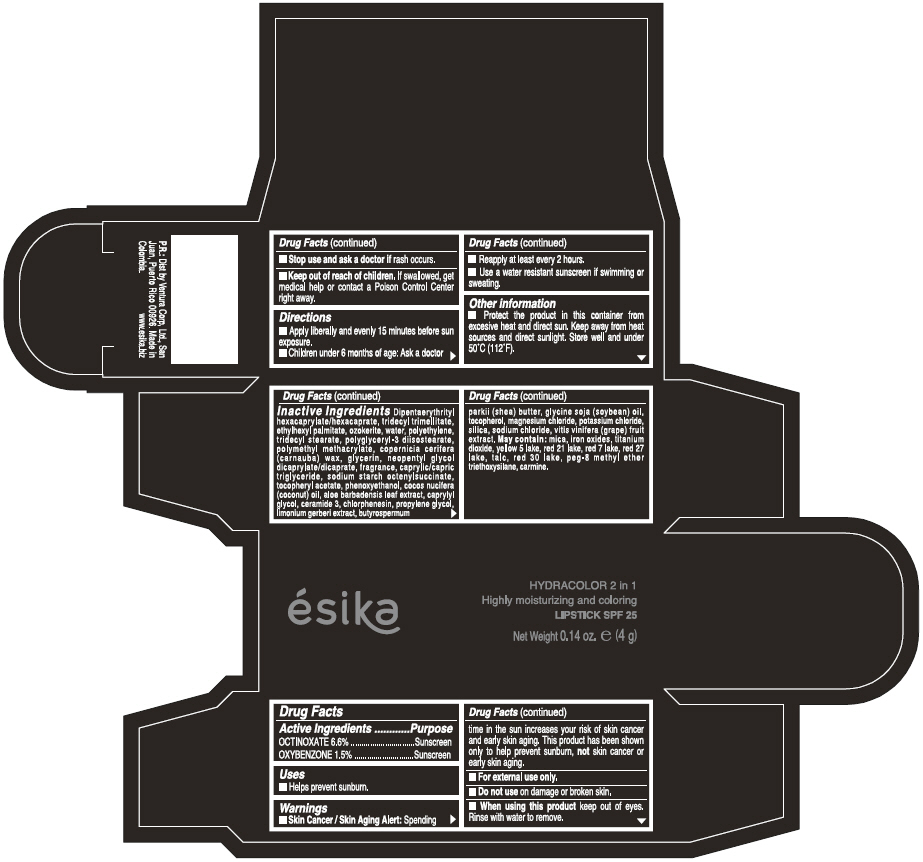
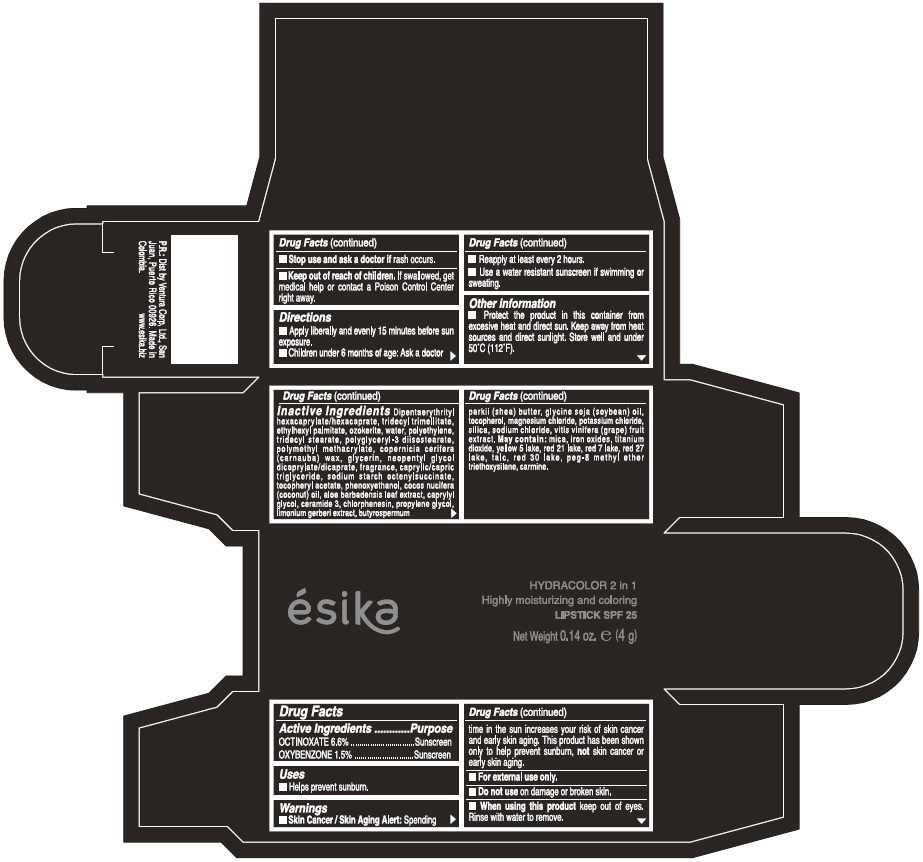
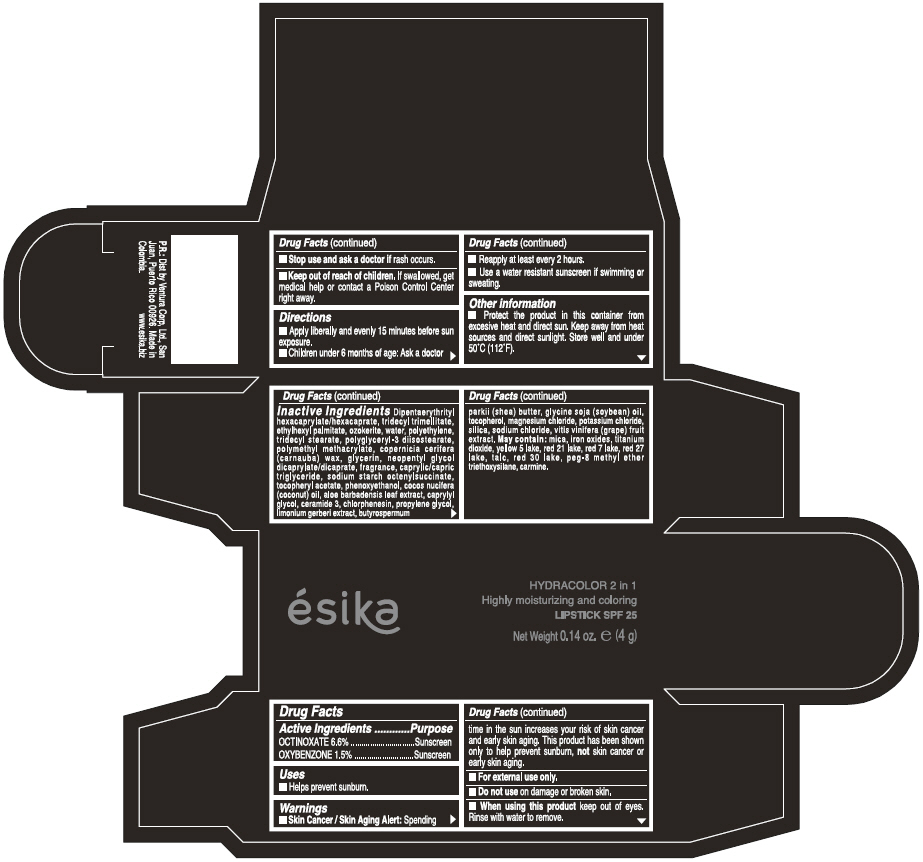
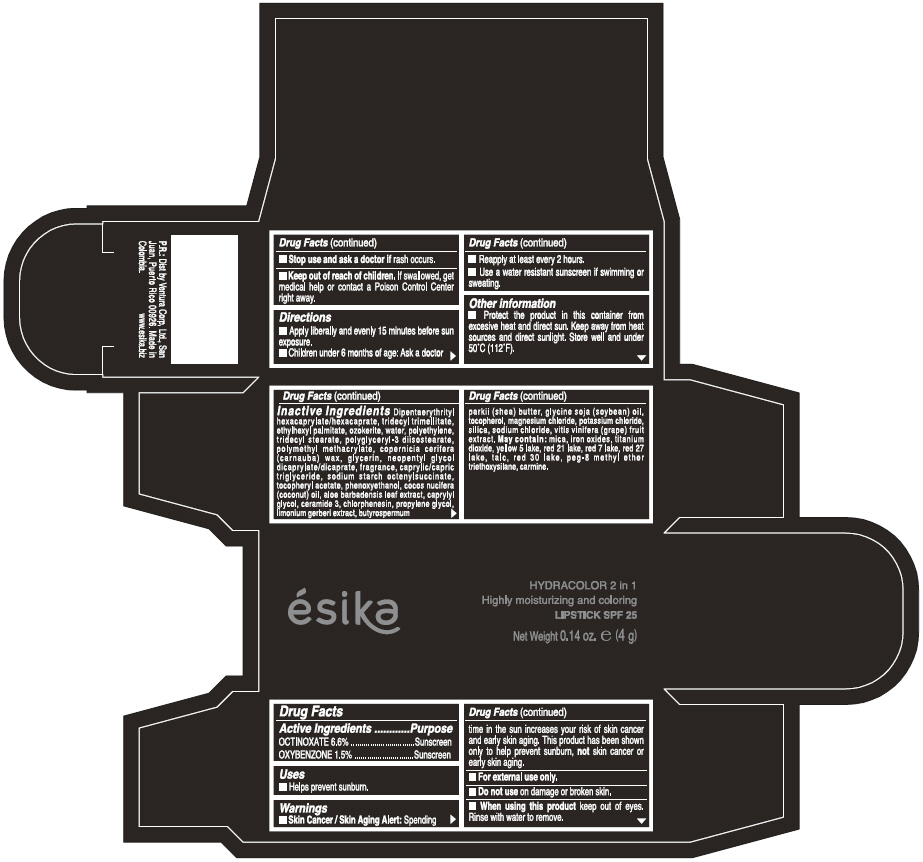
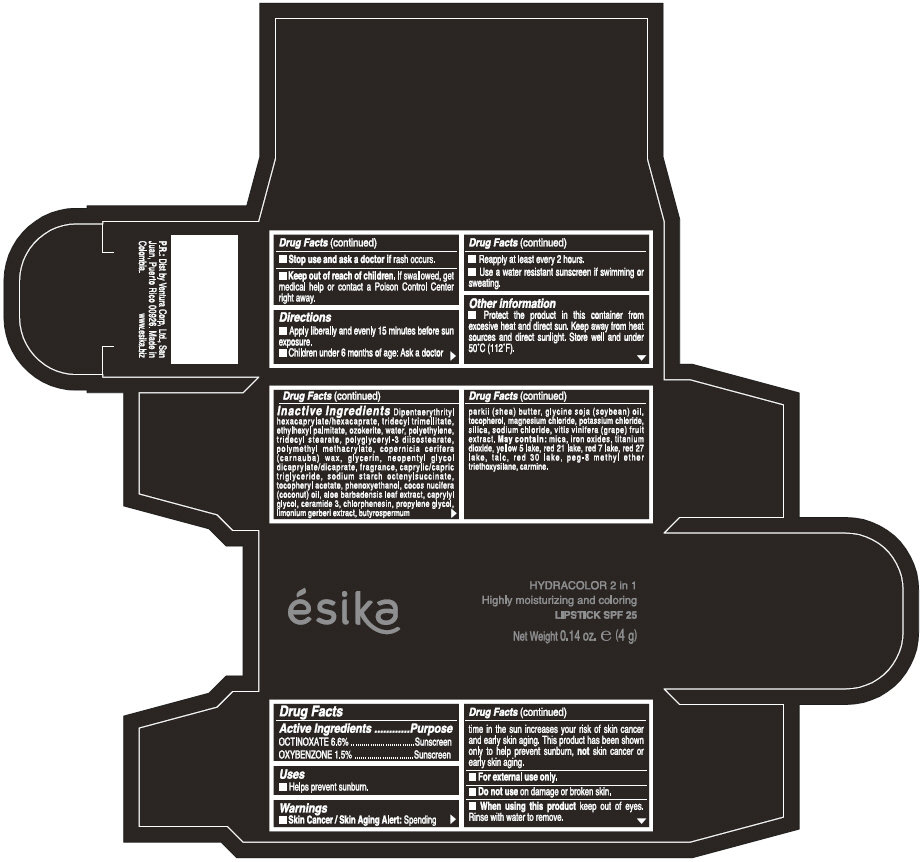
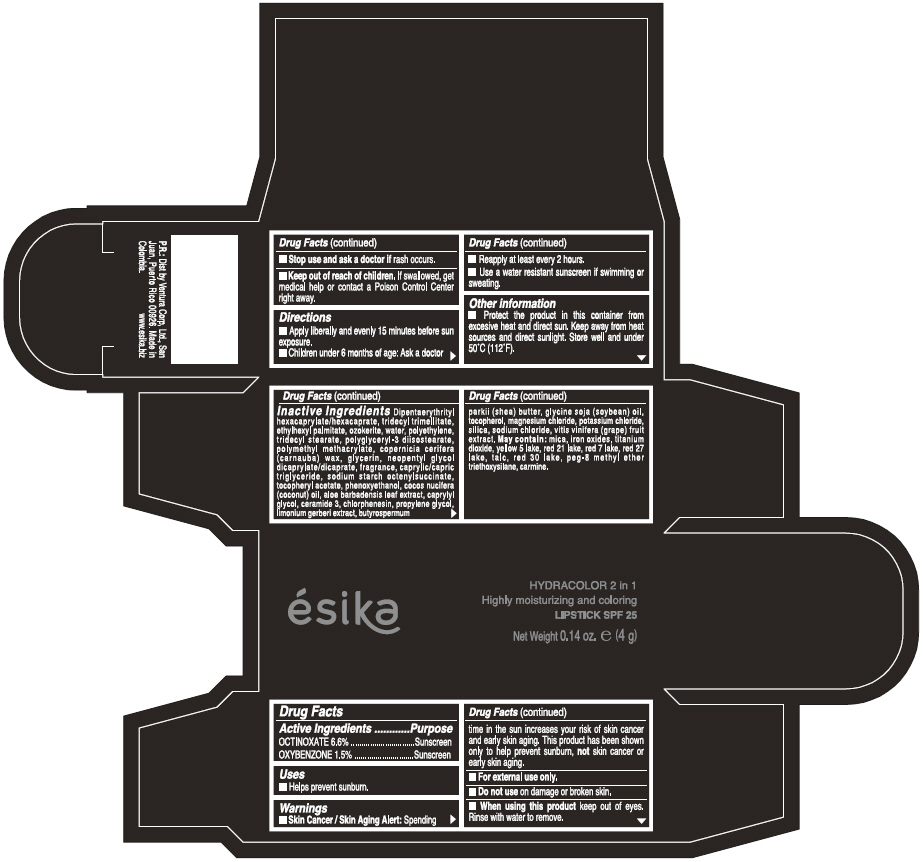
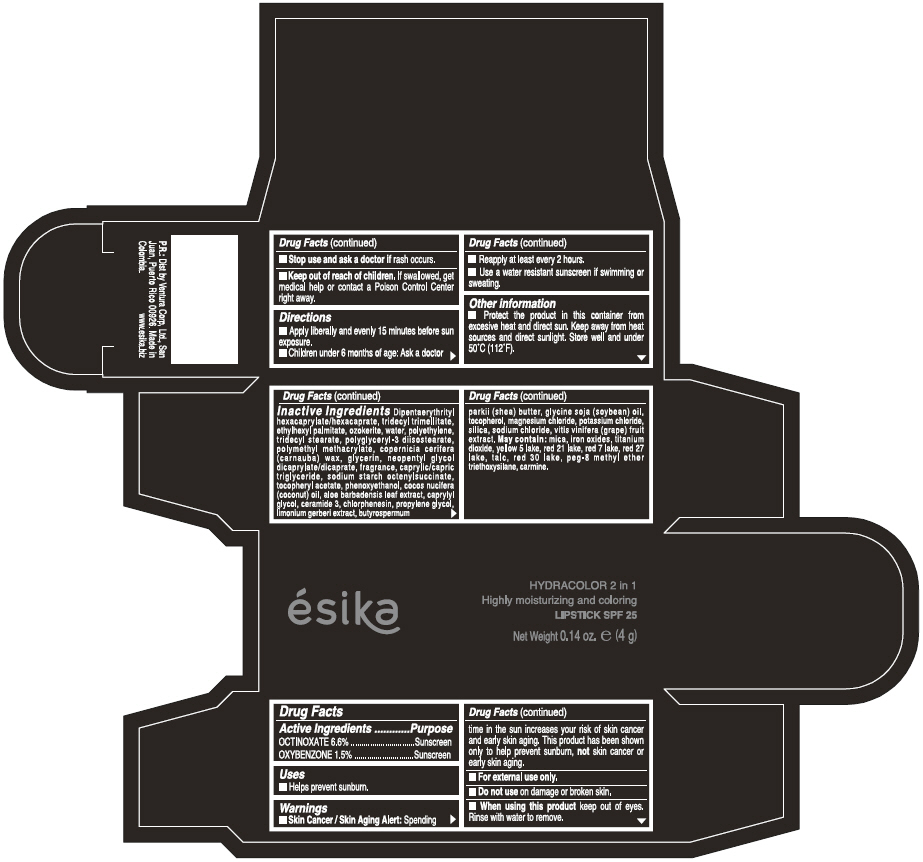
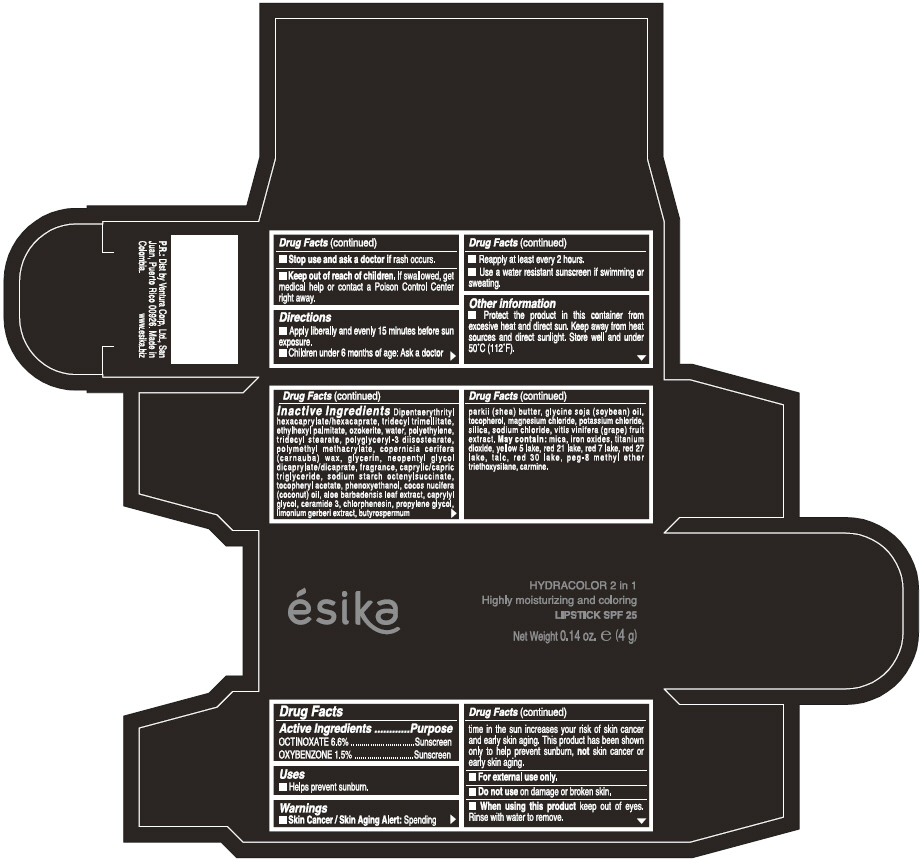
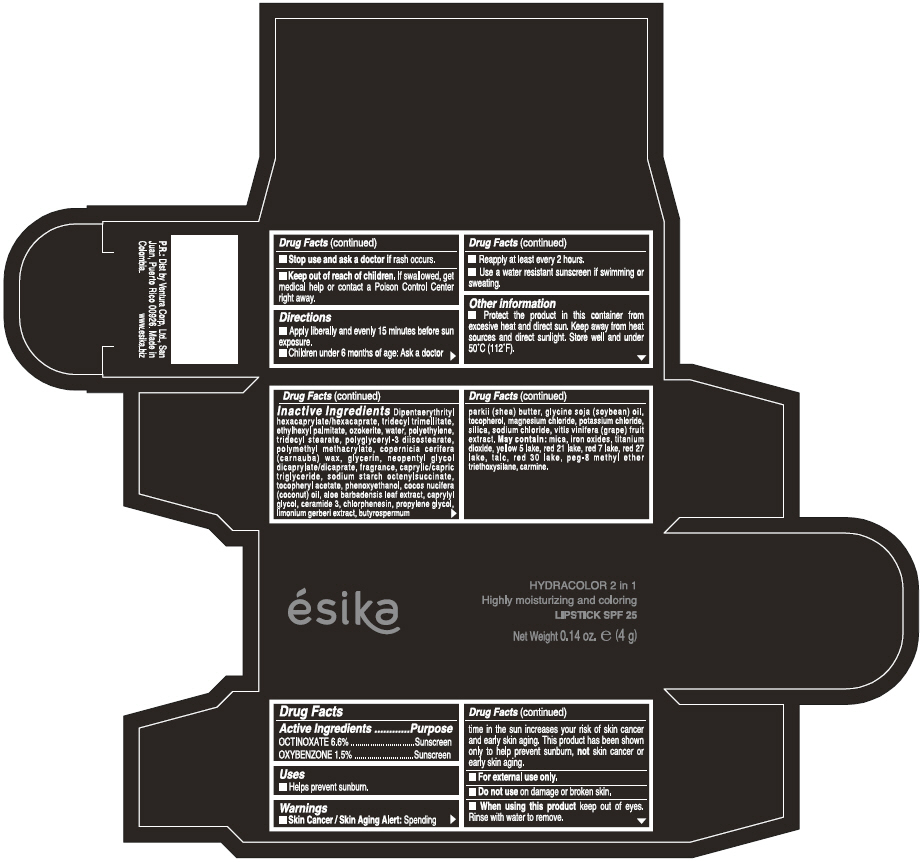
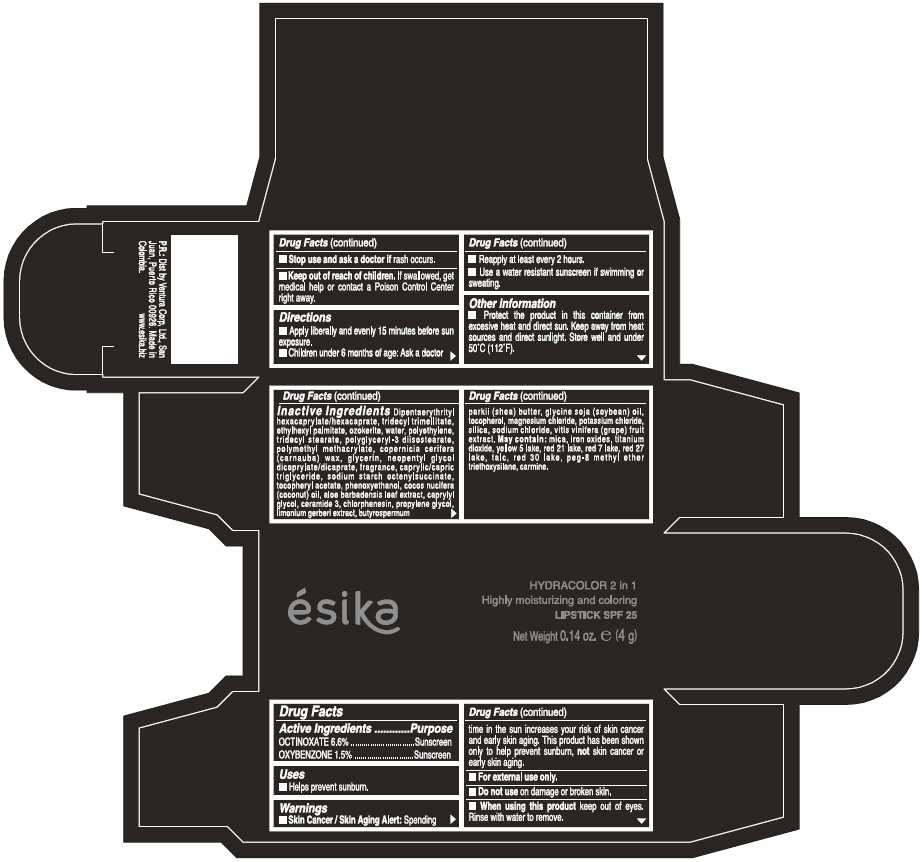
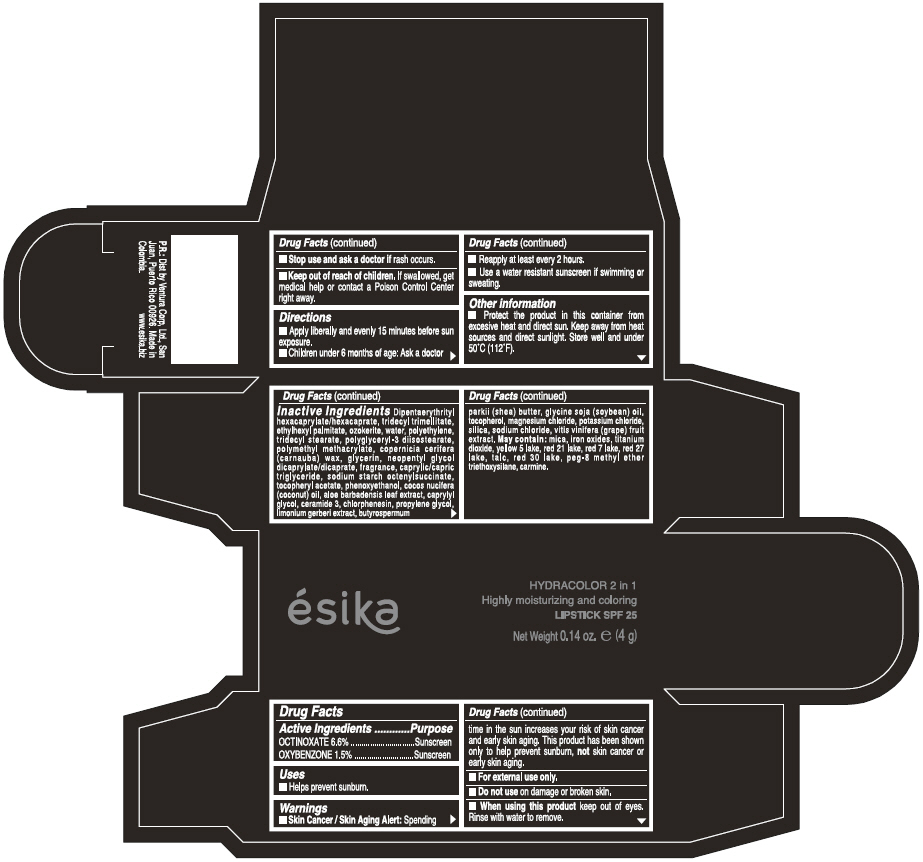
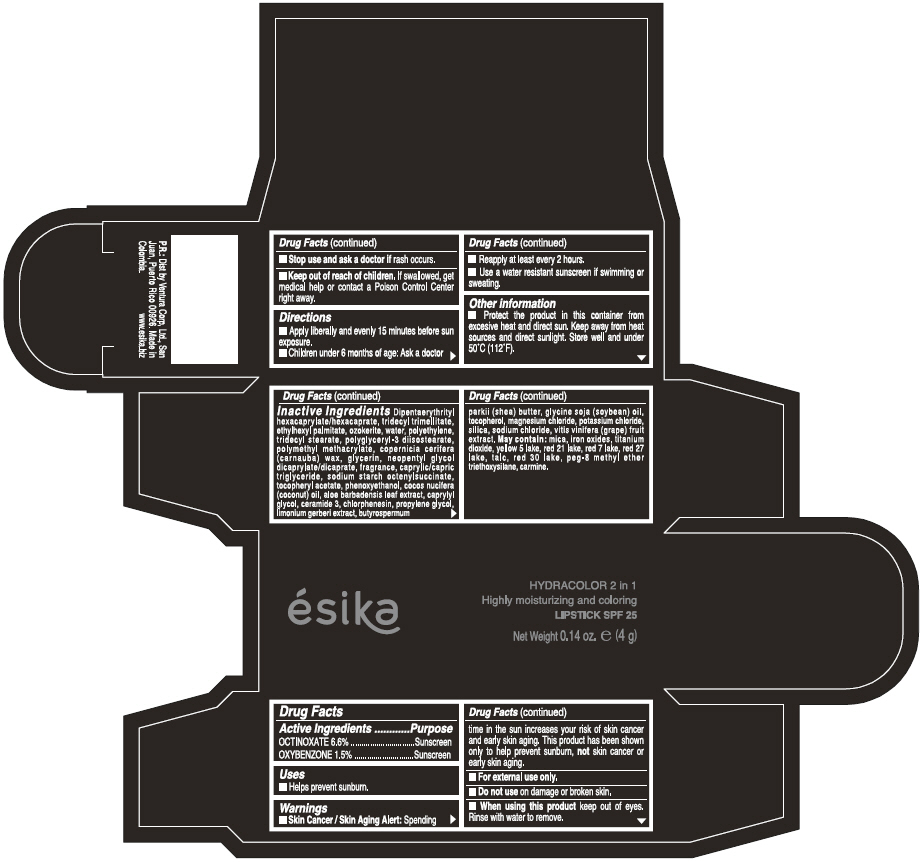
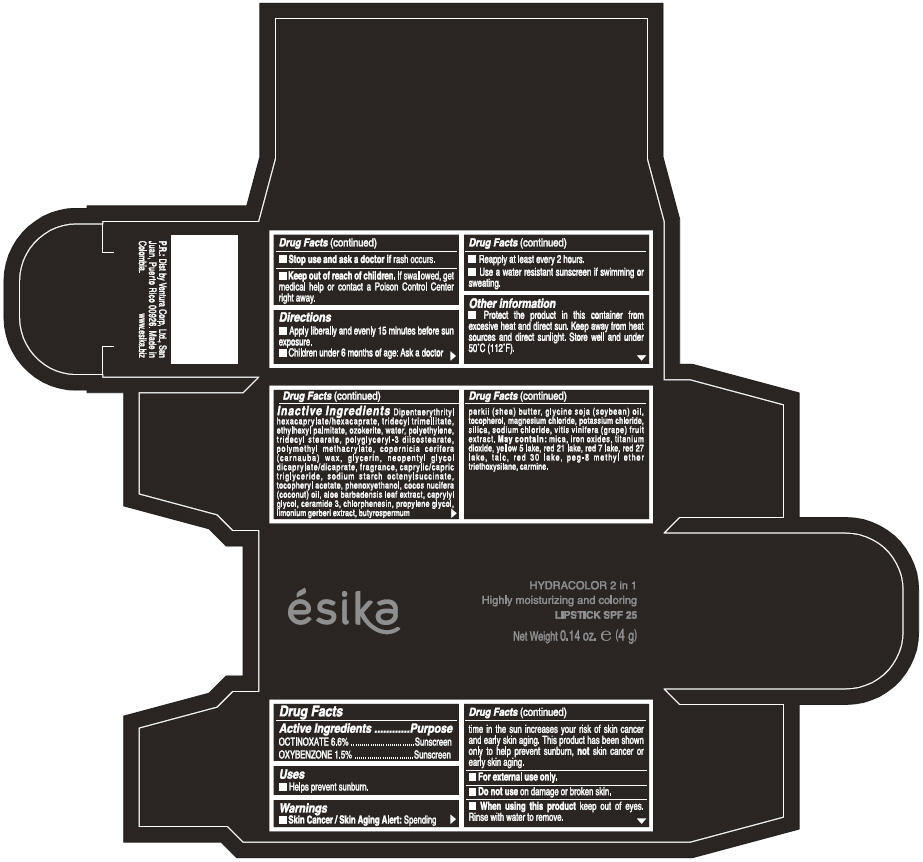
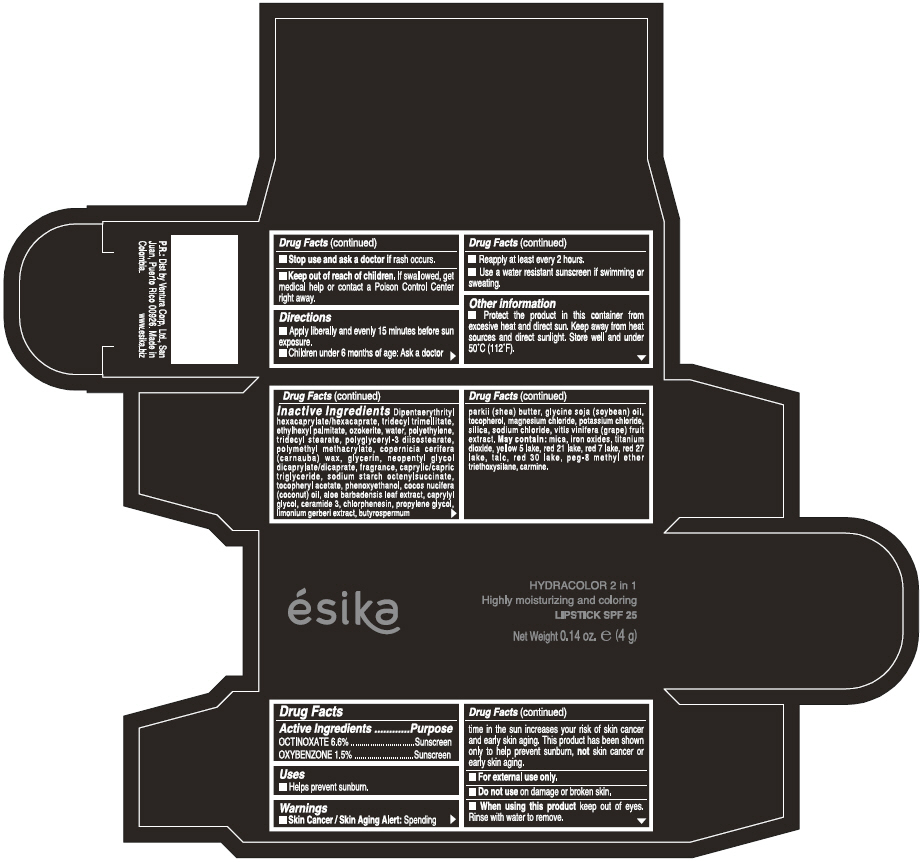
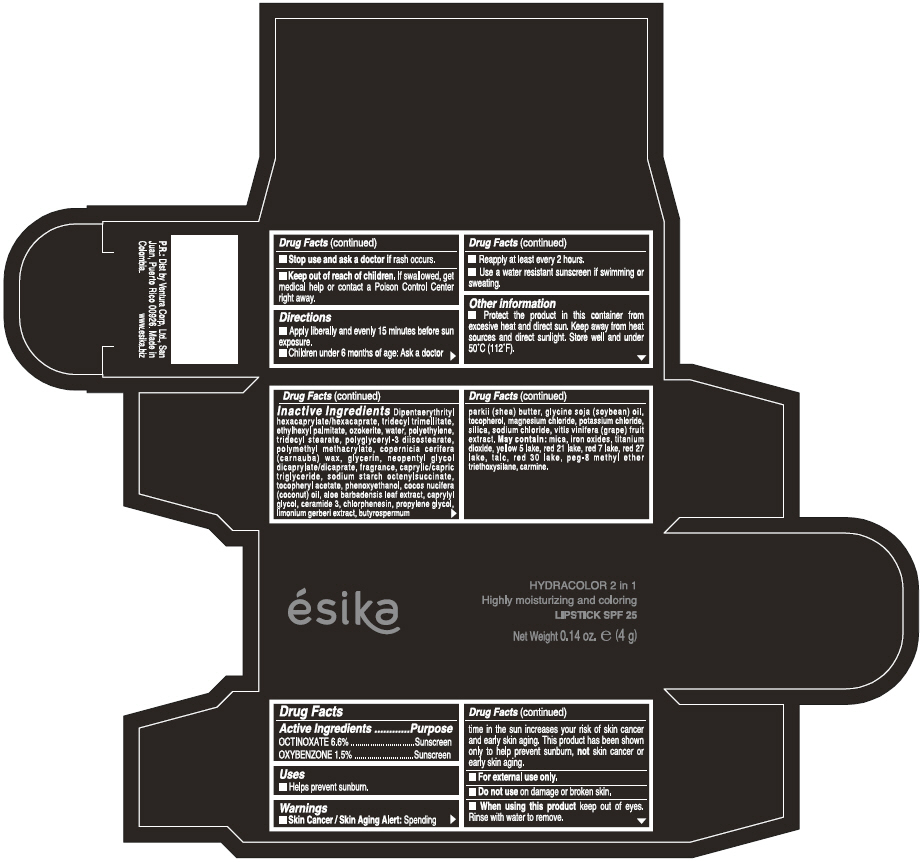
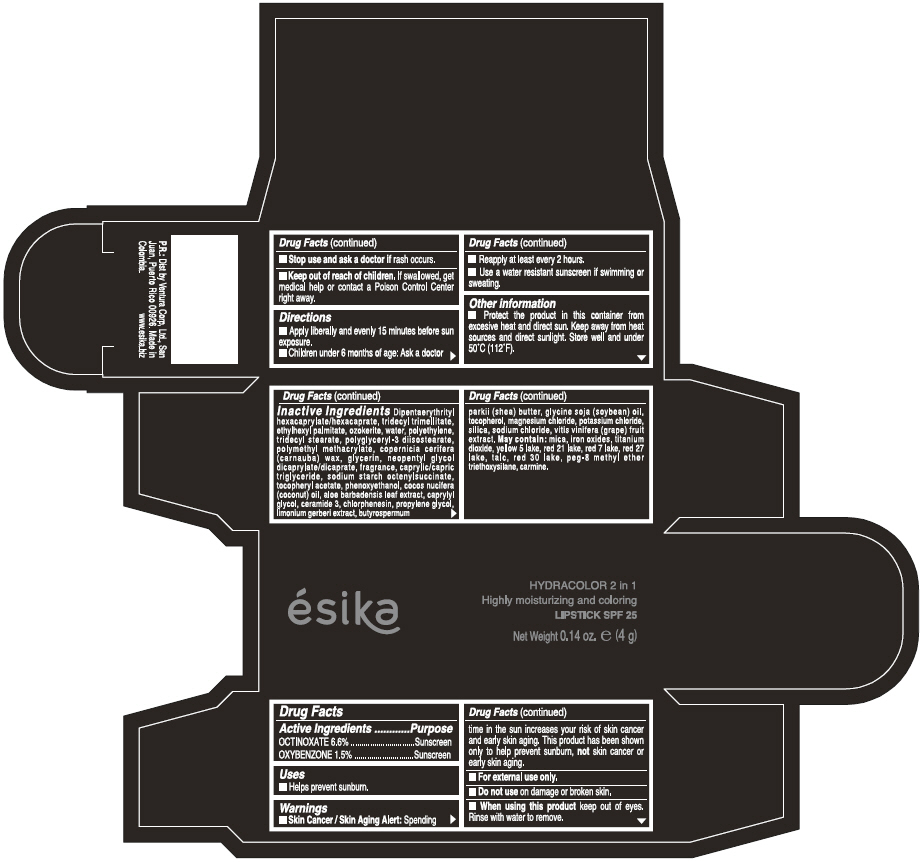
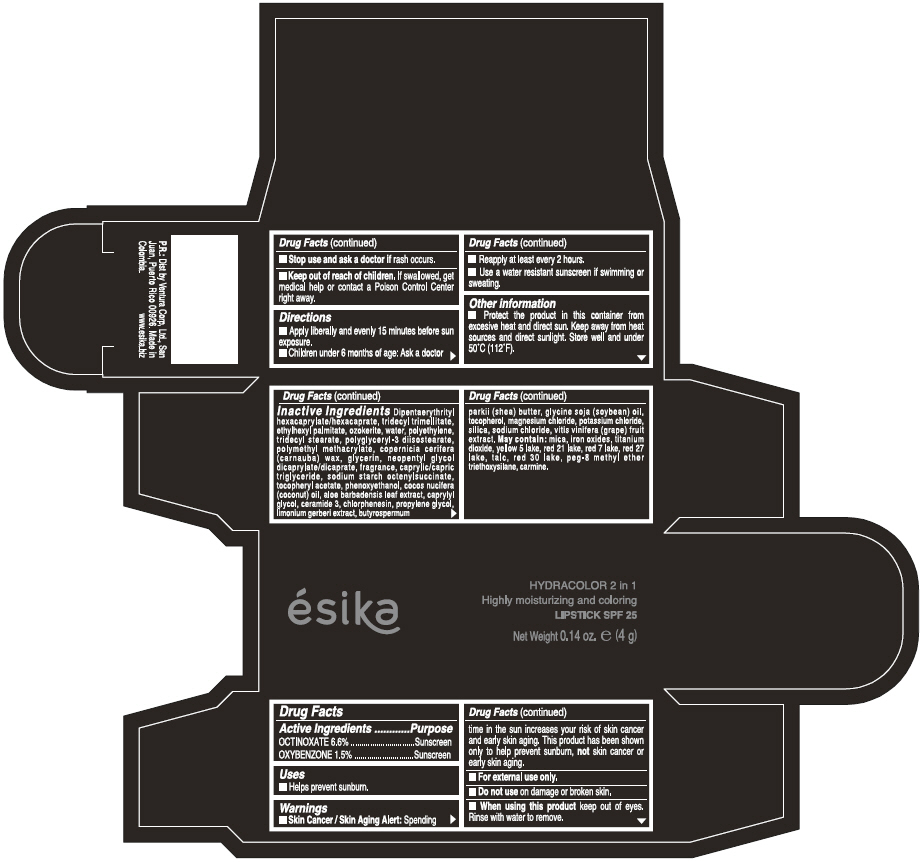
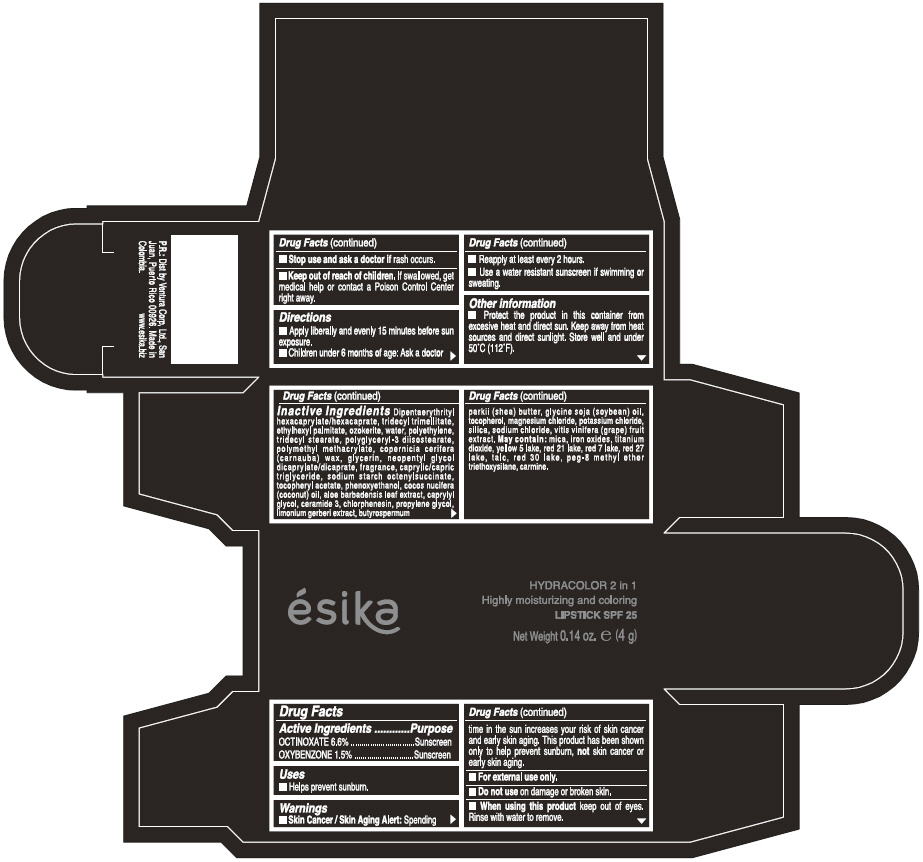
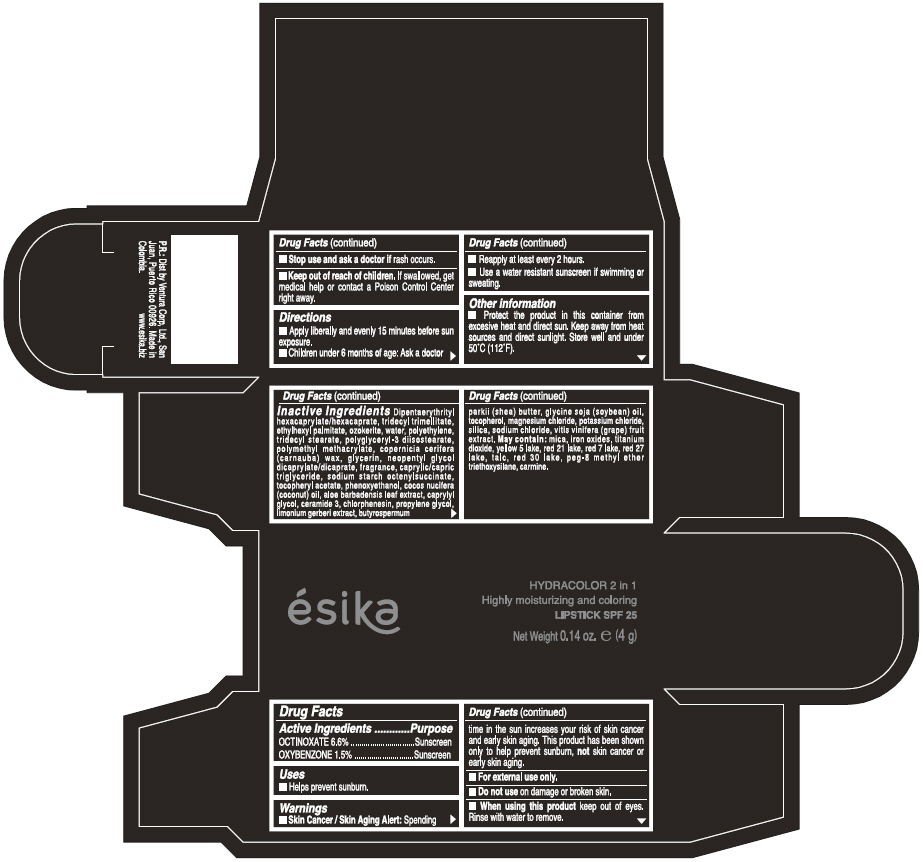
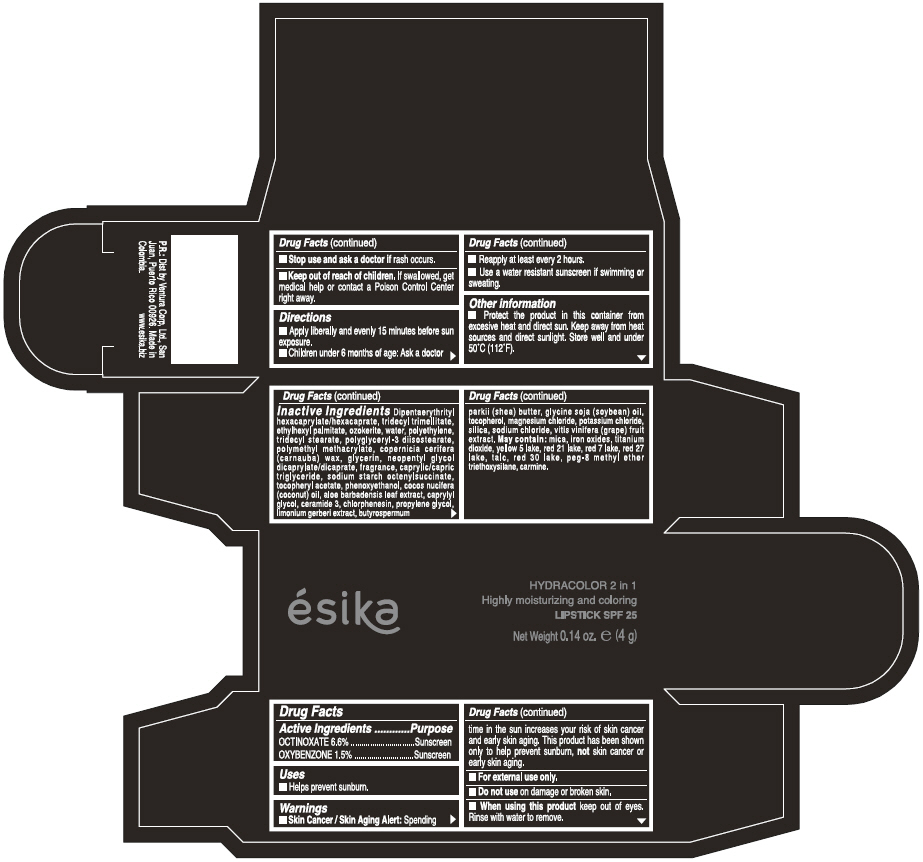
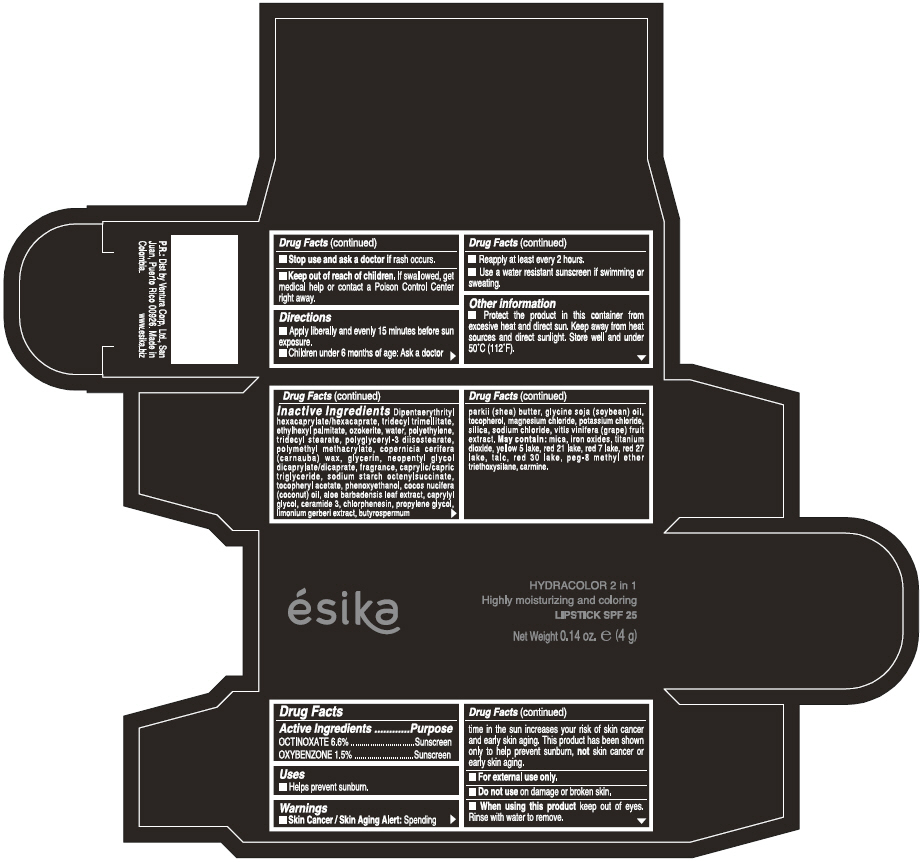
Label: ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (MARRON GLAMOUR) - BROWN- octinoxate and oxybenzone lipstick
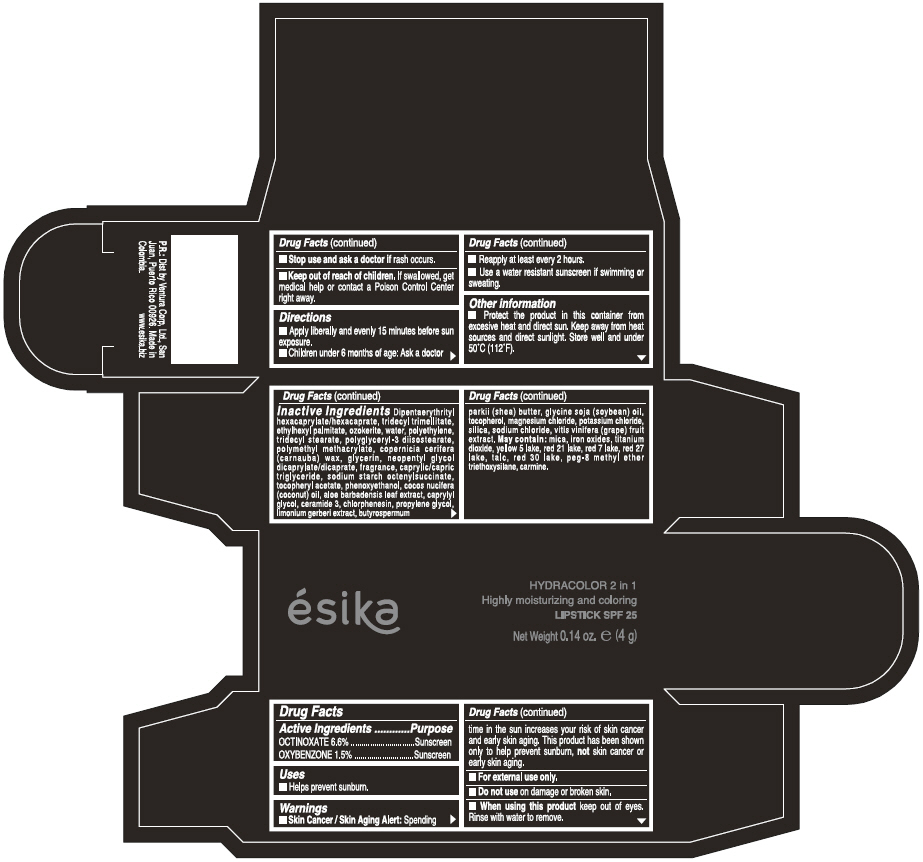
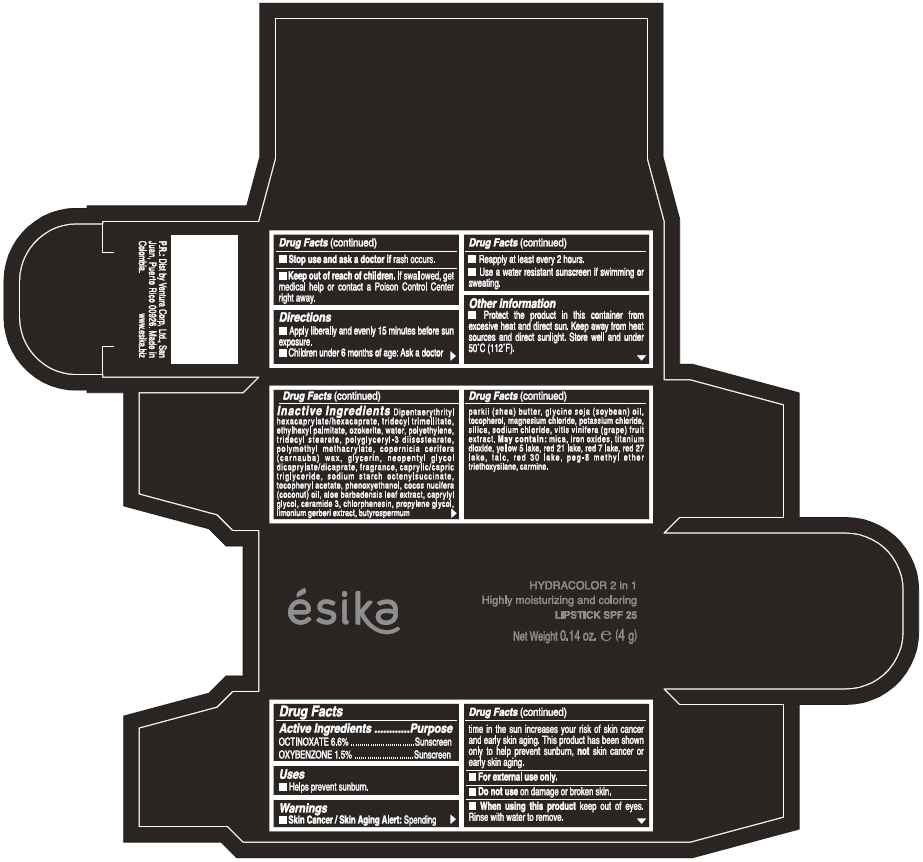
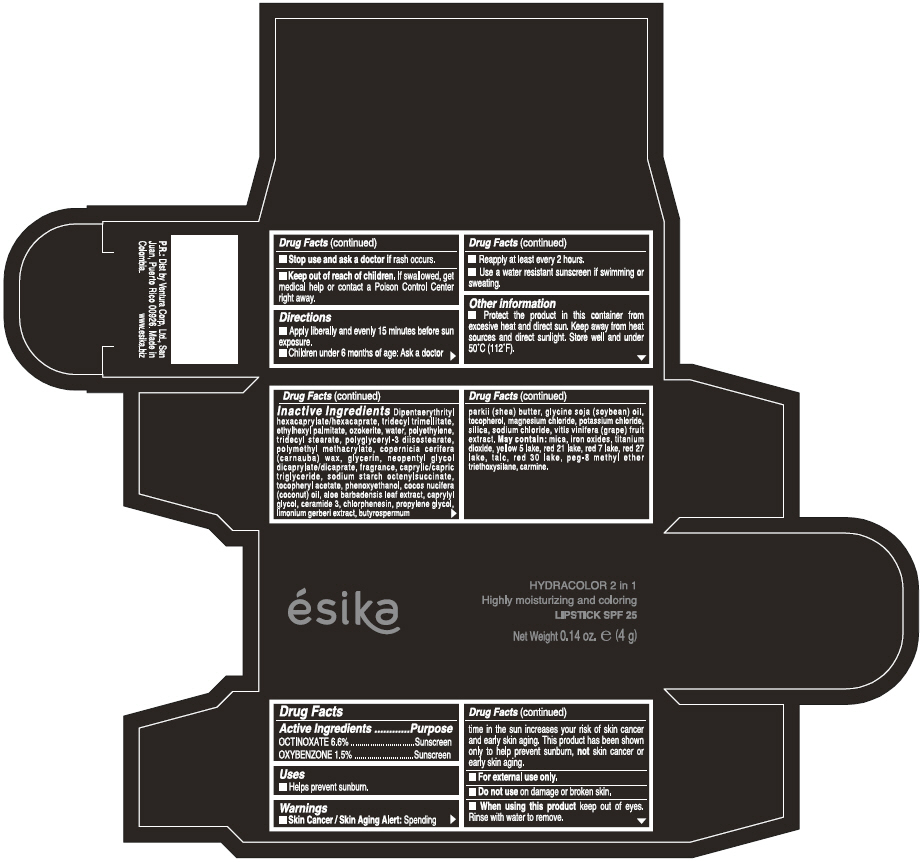
ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (ROJO DESEO) - RED- octinoxate and oxybenzone lipstick
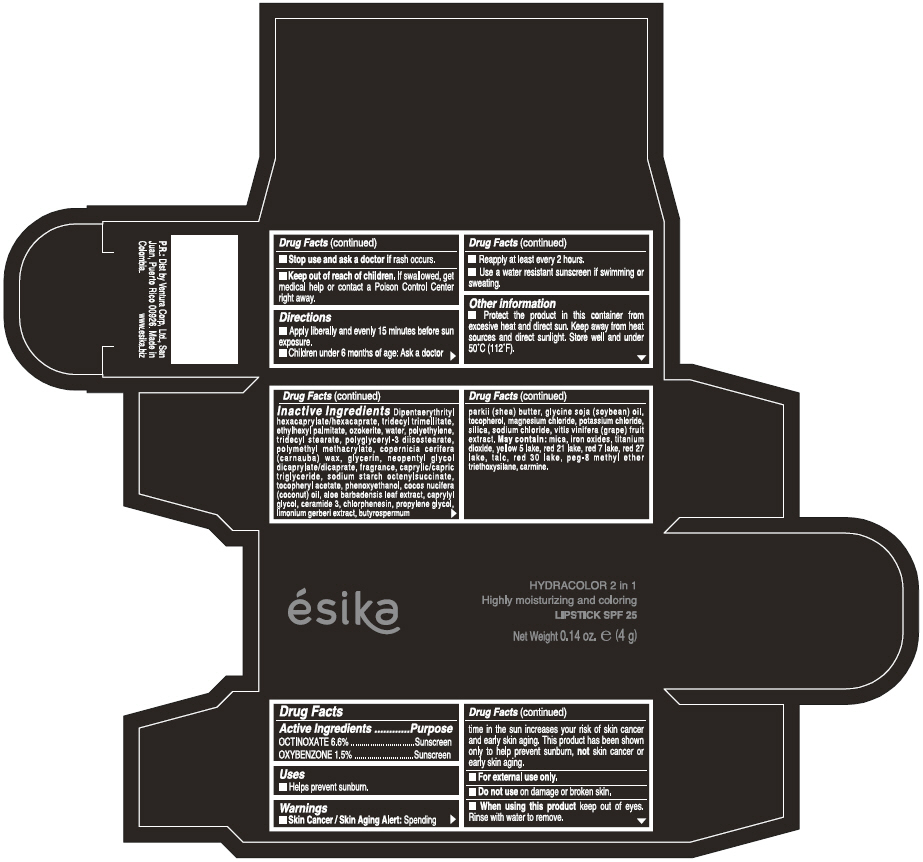
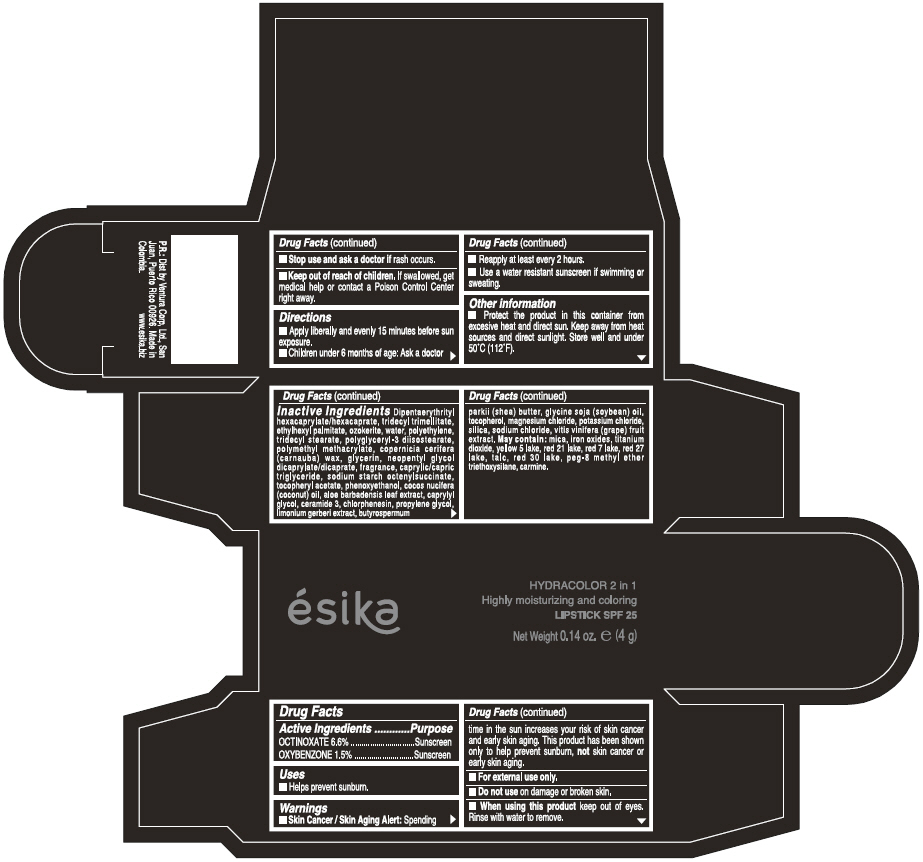
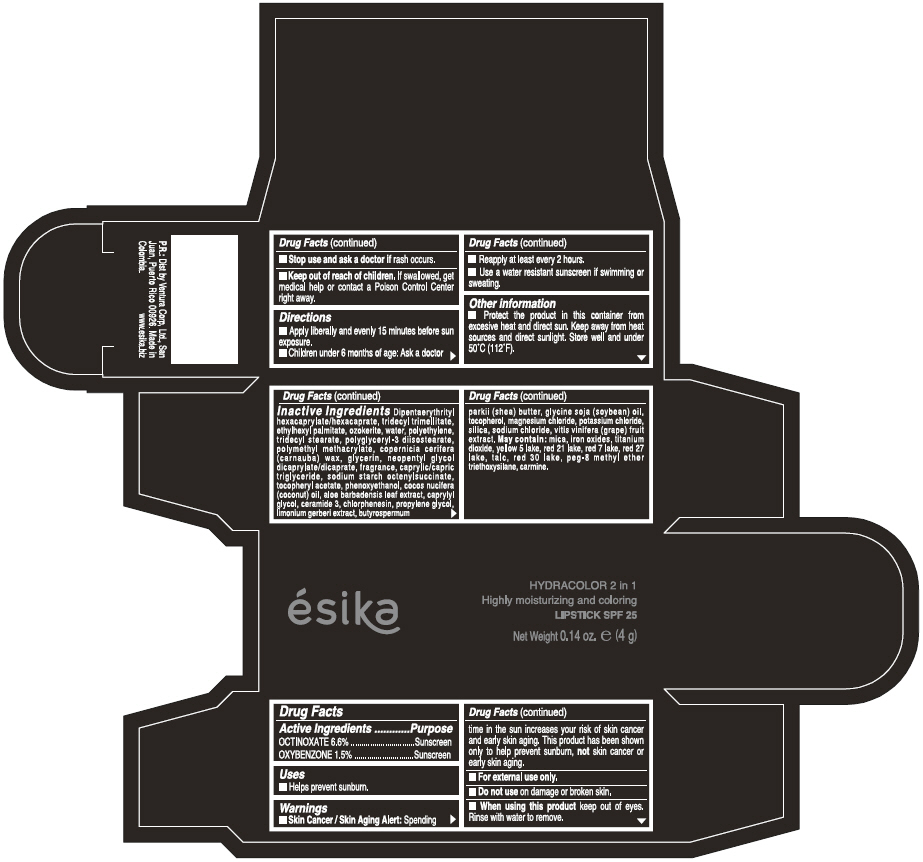
ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (ROJO INTRIGANTE) - RED- octinoxate and oxybenzone lipstick
-
Contains inactivated NDC Code(s)
NDC Code(s): 13537-916-01, 13537-916-02, 13537-917-03, 13537-917-04, view more13537-918-05, 13537-918-06, 13537-919-07, 13537-919-08, 13537-920-09, 13537-920-10, 13537-921-11, 13537-921-12, 13537-922-13, 13537-922-14, 13537-923-15, 13537-923-16, 13537-924-17, 13537-924-18, 13537-925-19, 13537-925-20, 13537-926-21, 13537-926-22, 13537-927-23, 13537-927-24, 13537-928-25, 13537-928-26, 13537-929-27, 13537-929-28, 13537-930-29, 13537-930-30, 13537-931-31, 13537-931-32, 13537-932-33, 13537-932-34, 13537-933-35, 13537-933-36, 13537-934-37, 13537-934-38, 13537-935-39, 13537-935-40, 13537-936-41, 13537-936-42, 13537-937-43, 13537-937-44, 13537-938-45, 13537-938-46, 13537-939-47, 13537-939-48 - Packager: Ventura Corporation LTD
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 1, 2014
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE, TRIDECYL TRIMELLITATE, ETHYLHEXYL PALMITATE, OZOKERITE, WATER, POLYETHYLENE, TRIDECYL STEARATE, POLYGLYCERYL-3 DIISOSTEARATE, POLYMETHYL METHACRYLATE, COPERNICIA CERIFERA (CARNAUBA) WAX, GLYCERIN, NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE, FRAGRANCE, CAPRYLIC/CAPRIC TRIGLYCERIDE, SODIUM STARCH OCTENYLSUCCINATE, TOCOPHERYL ACETATE, PHENOXYETHANOL, COCOS NUCIFERA (COCONUT) OIL, ALOE BARBADENSIS LEAF EXTRACT, CAPRYLYL GLYCOL, CERAMIDE 3, CHLORPHENESIN, PROPYLENE GLYCOL, LIMONIUM GERBERI EXTRACT, BUTYROSPERMUM PARKII (SHEA) BUTTER, GLYCINE SOJA OIL (GLYCINE SOJA (SOYBEAN) OIL), TOCOPHEROL, MAGNESIUM CHLORIDE, POTASSIUM CHLORIDE, SILICA, SODIUM CHLORIDE,VITIS VINIFERA (GRAPE) FRUIT EXTRACT
May contain:
MICA,IRON OXIDES, TITANIUM DIOXIDE, YELLOW 5 LAKE, RED 21 LAKE, RED 7 LAKE, RED 27 LAKE, TALC, RED 30 LAKE, PEG-8 METHYL ETHER TRIETHOXYSILANE, CARMINE. - SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (MARRON GLAMOUR) - BROWN
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROJO DESEO) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROJO INTRIGANTE) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROJO LUJURIA) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (CORAL CHIC) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROSA ENCANTO) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (BORGONA SEXY) - PURPLE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (FUCSIA CAUTIVANTE) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (GRANATE SEDUCTOR) - PURPLE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROSA ROMANTICA) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (NARANJA PRIMAVERA) - ORANGE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (FRAMBUESA MANIA) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROSA DULZURA) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (PIMIENTA CALIENTE) - BROWN
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (CANELA NUDE) - ORANGE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (MORA GLAM) - BROWN
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (CORAL INTENSE) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (FRESA COQUETA) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (PIMIENTA CALIENTE FEMME) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (GRANATE SEDUCTOR FEMME) - PURPLE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (FUCSIA CAUTIVANTE FEMME) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROSA ROMANTICA FEMME) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROJO INTRIGANTE FEMME) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROSA ENCANTO FEMME) - PINK
-
INGREDIENTS AND APPEARANCE
ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (MARRON GLAMOUR) - BROWN
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-916 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-916-02 1 in 1 BOX 1 NDC:13537-916-01 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (ROJO DESEO) - RED
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-917 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-917-04 1 in 1 BOX 1 NDC:13537-917-03 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (ROJO INTRIGANTE) - RED
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-918 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-918-06 1 in 1 BOX 1 NDC:13537-918-05 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (ROJO LUJURIA) - RED
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-919 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-919-08 1 in 1 BOX 1 NDC:13537-919-07 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (CORAL CHIC) - PINK
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-920 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-920-10 1 in 1 BOX 1 NDC:13537-920-09 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (ROSA ENCANTO) - PINK
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-921 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-921-12 1 in 1 BOX 1 NDC:13537-921-11 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (BORGONA SEXY) - PURPLE
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-922 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-922-14 1 in 1 BOX 1 NDC:13537-922-13 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (FUCSIA CAUTIVANTE) - PINK
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-923 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-923-16 1 in 1 BOX 1 NDC:13537-923-15 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (GRANATE SEDUCTOR) - PURPLE
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-924 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-924-18 1 in 1 BOX 1 NDC:13537-924-17 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (ROSA ROMANTICA) - PINK
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-925 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-925-20 1 in 1 BOX 1 NDC:13537-925-19 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (NARANJA PRIMAVERA) - ORANGE
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-926 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-926-22 1 in 1 BOX 1 NDC:13537-926-21 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (FRAMBUESA MANIA) - RED
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-927 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-927-24 1 in 1 BOX 1 NDC:13537-927-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (ROSA DULZURA) - PINK
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-928 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-928-26 1 in 1 BOX 1 NDC:13537-928-25 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (PIMIENTA CALIENTE) - BROWN
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-929 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-929-28 1 in 1 BOX 1 NDC:13537-929-27 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (CANELA NUDE) - ORANGE
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-930 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-930-30 1 in 1 BOX 1 NDC:13537-930-29 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (MORA GLAM) - BROWN
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-931 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-931-32 1 in 1 BOX 1 NDC:13537-931-31 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (CORAL INTENSE) - RED
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-932 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-932-34 1 in 1 BOX 1 NDC:13537-932-33 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (FRESA COQUETA) - RED
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-933 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-933-36 1 in 1 BOX 1 NDC:13537-933-35 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (PIMIENTA CALIENTE FEMME) - RED
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-934 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-934-38 1 in 1 BOX 1 NDC:13537-934-37 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (GRANATE SEDUCTOR FEMME) - PURPLE
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-935 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-935-40 1 in 1 BOX 1 NDC:13537-935-39 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (FUCSIA CAUTIVANTE FEMME) - PINK
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-936 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-936-42 1 in 1 BOX 1 NDC:13537-936-41 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (ROSA ROMANTICA FEMME) - PINK
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-937 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-937-44 1 in 1 BOX 1 NDC:13537-937-43 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (ROJO INTRIGANTE FEMME) - RED
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-938 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-938-46 1 in 1 BOX 1 NDC:13537-938-45 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 ESIKA HYDRACOLOR 2 IN 1 HIGHLY MOISTURIZING AND COLORING SPF 25 (ROSA ENCANTO FEMME) - PINK
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-939 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE 3 (UNII: 4370DF050B) CHLORPHENESIN (UNII: I670DAL4SZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) WINE GRAPE (UNII: 3GOV20705G) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 27 (UNII: 2LRS185U6K) TALC (UNII: 7SEV7J4R1U) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-939-48 1 in 1 BOX 1 NDC:13537-939-47 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/30/2014 Labeler - Ventura Corporation LTD (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE(13537-916, 13537-917, 13537-918, 13537-919, 13537-920, 13537-921, 13537-922, 13537-923, 13537-924, 13537-925, 13537-926, 13537-927, 13537-928, 13537-929, 13537-930, 13537-931, 13537-932, 13537-933, 13537-934, 13537-935, 13537-936, 13537-937, 13537-938, 13537-939)