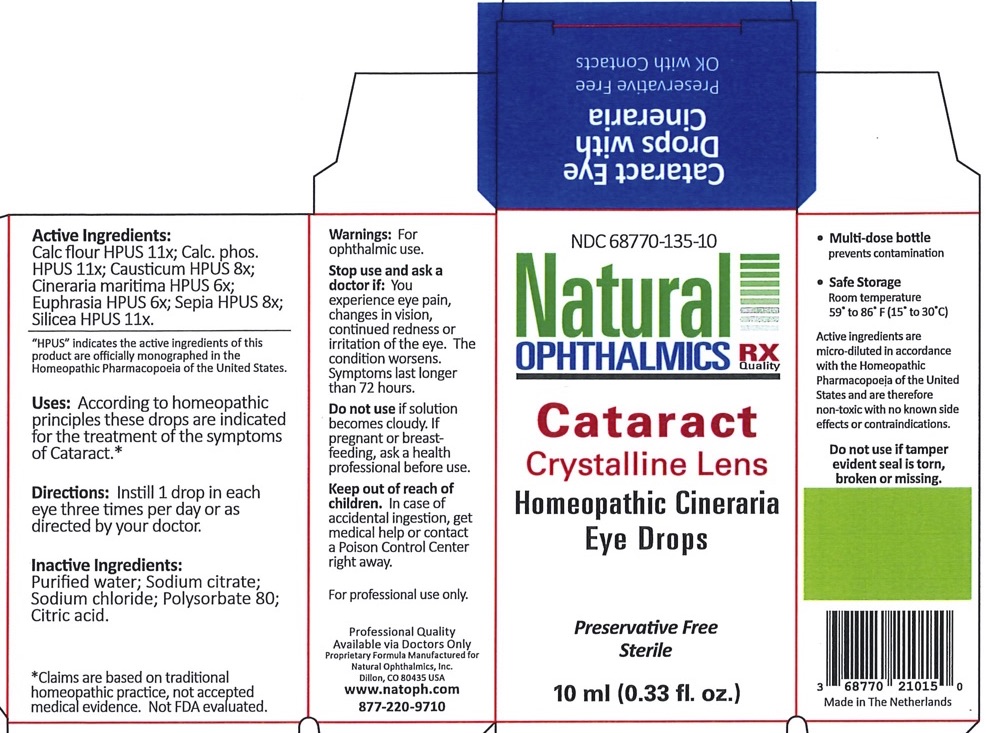
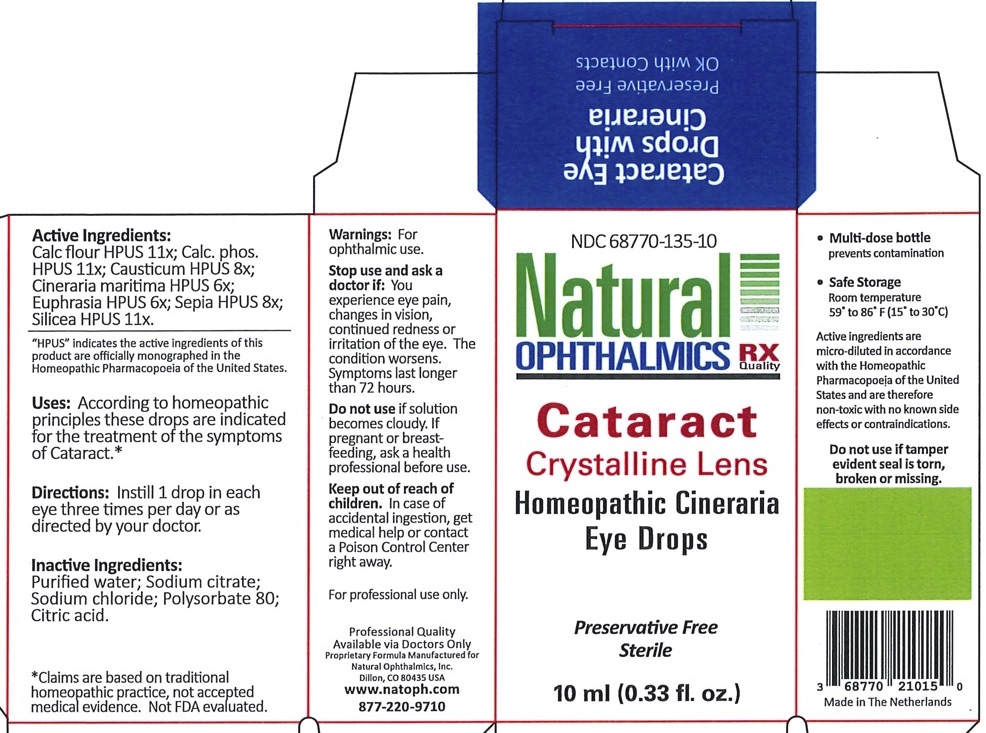
Label: CATARACT CRYSTALLINE LENS- cineraria maritima, euphrasia strictica, causticum, calcarea phosphorica, sepia, calcarea flourica, silicea liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 68770-135-10 - Packager: Natural Ophthalmics, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 14, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Homeopathic Purpose
Cineraria Maritima HPUS 6X Increases circulation in the intraocular tissues Euphrasia (Eyebright) HPUS 6X General dryness, redness and irritation of the eye and lids Causticum HPUS 8X Eye pain Calcarea phosphorica HPUS 11X Blurry vision, eye pain from reading Sepia HPUS 8X Glares, black spots, veils, points, sparks, flashes and streaks of light Calcarea fluorica HPUS 11X Cataract, corneal opacities, keratitis, flickering and floaters Silicea HPUS 11X Spotted vision, cataract, opacities, keratitis *Claims are based on traditional homeopathic practice, not accepted medicalevidence. Not FDA evaluated
- Active Ingredients
- Inactive Ingredients
- Uses
- Directions
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- If pregnant or breast feeding
- Stop use and ask a doctor if:
- Do not use
- Storage
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CATARACT CRYSTALLINE LENS
cineraria maritima, euphrasia strictica, causticum, calcarea phosphorica, sepia, calcarea flourica, silicea liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68770-135 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength JACOBAEA MARITIMA (UNII: U4B223LS4X) (JACOBAEA MARITIMA - UNII:U4B223LS4X) JACOBAEA MARITIMA 6 [hp_X] in 1 mL EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 6 [hp_X] in 1 mL CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 8 [hp_X] in 1 mL TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (PHOSPHATE ION - UNII:NK08V8K8HR) TRIBASIC CALCIUM PHOSPHATE 11 [hp_X] in 1 mL SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 8 [hp_X] in 1 mL CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 11 [hp_X] in 1 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 11 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68770-135-10 1 in 1 PACKAGE 07/14/2020 1 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/14/2020 Labeler - Natural Ophthalmics, Inc (118039333)