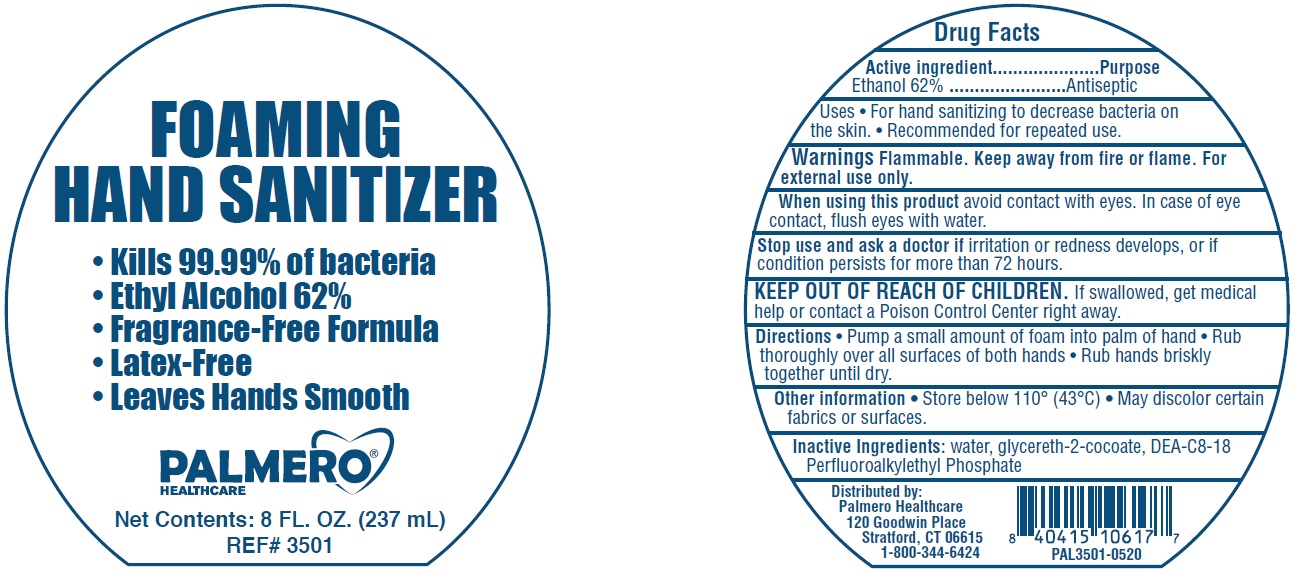
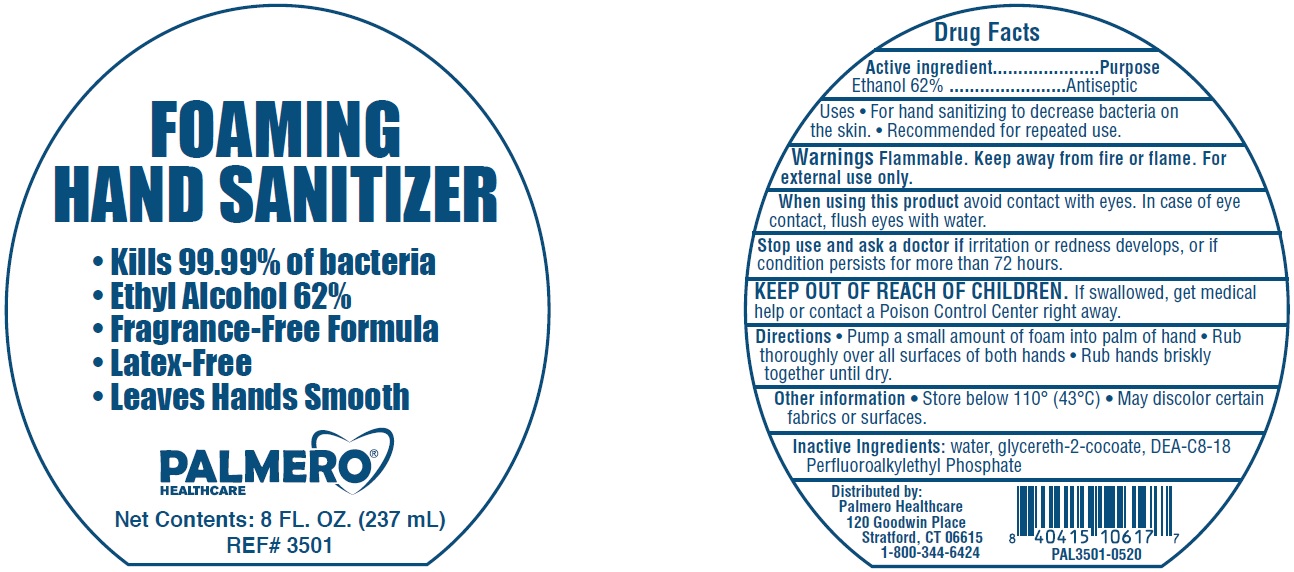
Label: PALMERO HEALTHCARE FOAMING HAND SANITIZER- alcohol liquid
- NDC Code(s): 74545-001-08
- Packager: Palmero Healthcare
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive Ingredients:
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
PALMERO HEALTHCARE FOAMING HAND SANITIZER
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74545-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.62 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERETH-2 COCOATE (UNII: JWM00VS7HC) DIETHANOLAMINE BIS(C8-C18 PERFLUOROALKYLETHYL)PHOSPHATE (UNII: 4J55VM509S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74545-001-08 237 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 06/17/2020 Labeler - Palmero Healthcare (080530675)