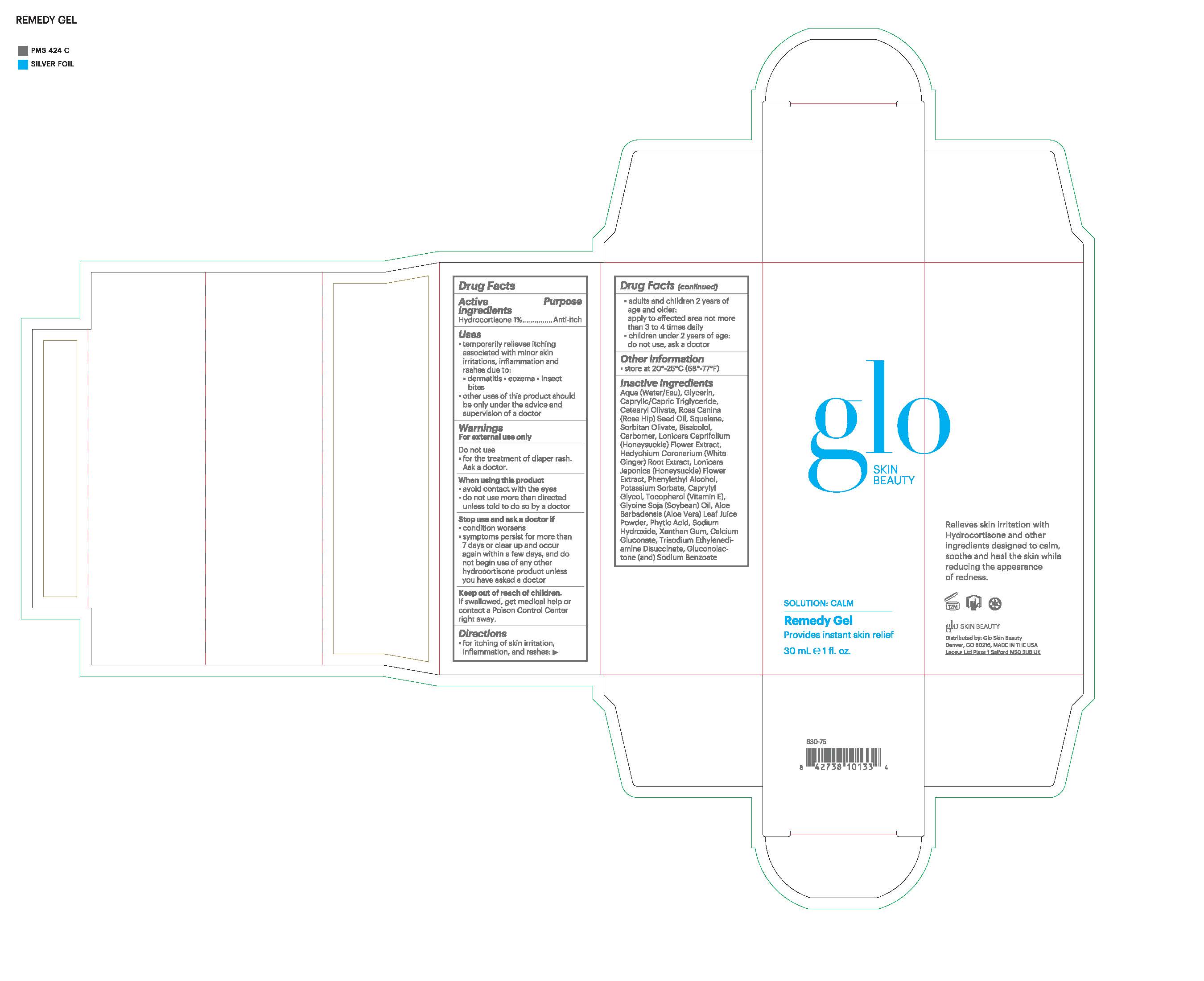
Label: GLO SKIN BEAUTY REMEDY GEL gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 60541-1900-3 - Packager: Hayden Caleel LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 28, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- for the treatment of iaper rash. Ask a doctor
When using this product
• avoid contact with the eyes
• do not use more than directed unless told to do so by a doctorStop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days, and do not begin use of any other hyrdrocortisone product unless you have asked a doctor
- Keep out of reach of children
- Directions
-
Inactive Ingredients
Aqua (Water), Glycerin, Caprylic/Capric Triglyceride, Cetearyl Olivate, Rosa Canina (Rose Hip) Fruit Oil, Squalane, Sorbitan Olivate, Bisabolol, Carbomer, Gluconolactone, Lonicera Caprifolium (Honeysuckle) Flower Extract, Hedychium Coronarium (White Ginger) Root Extract, Lonicera Japonica (Honeysuckle) Flower Extract, Sodium Benzoate, Phenethyl Alcohol, Potassium Sorbate, Caprylyl Glycol, Tocopherol, Glycine Soja (Soybean) Oil, Aloe Barbadensis Leaf Juice Powder, Phytic Acid, Sodium Hydroxide, Xanthan Gum, Trisodium Ethylenediamine Disuccinate
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
GLO SKIN BEAUTY REMEDY GEL
glo skin beauty remedy gel gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60541-1900 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CAPRYLIC/CAPRIC ACID (UNII: DI775RT244) ROSA CANINA FRUIT OIL (UNII: CR7307M3QZ) SQUALANE (UNII: GW89575KF9) SORBITAN OLIVATE (UNII: MDL271E3GR) .ALPHA.-BISABOLOL, (+)- (UNII: 105S6I733Z) CARBOMER 940 (UNII: 4Q93RCW27E) GLUCONOLACTONE (UNII: WQ29KQ9POT) LONICERA CAPRIFOLIUM FLOWER (UNII: 5N1WD9784U) HEDYCHIUM CORONARIUM ROOT (UNII: 92A6N0IQN9) LONICERA JAPONICA FLOWER (UNII: 4465L2WS4Y) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CAPRYLYL GLYCOL (UNII: 00YIU5438U) TOCOPHEROL (UNII: R0ZB2556P8) SOYBEAN OIL (UNII: 241ATL177A) ALOE VERA WHOLE (UNII: KIZ4X2EHYX) SODIUM HYDRIDE (UNII: 23J3BHR95O) XANTHAN GUM (UNII: TTV12P4NEE) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60541-1900-3 1 in 1 CARTON 12/31/2017 1 30 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 12/31/2017 Labeler - Hayden Caleel LLC (011367468) Registrant - Hayden Caleel LLC (011367468) Establishment Name Address ID/FEI Business Operations Island Kinetics, Inc. d.b.a. CoValence Laboratories 959735002 manufacture(60541-1900)