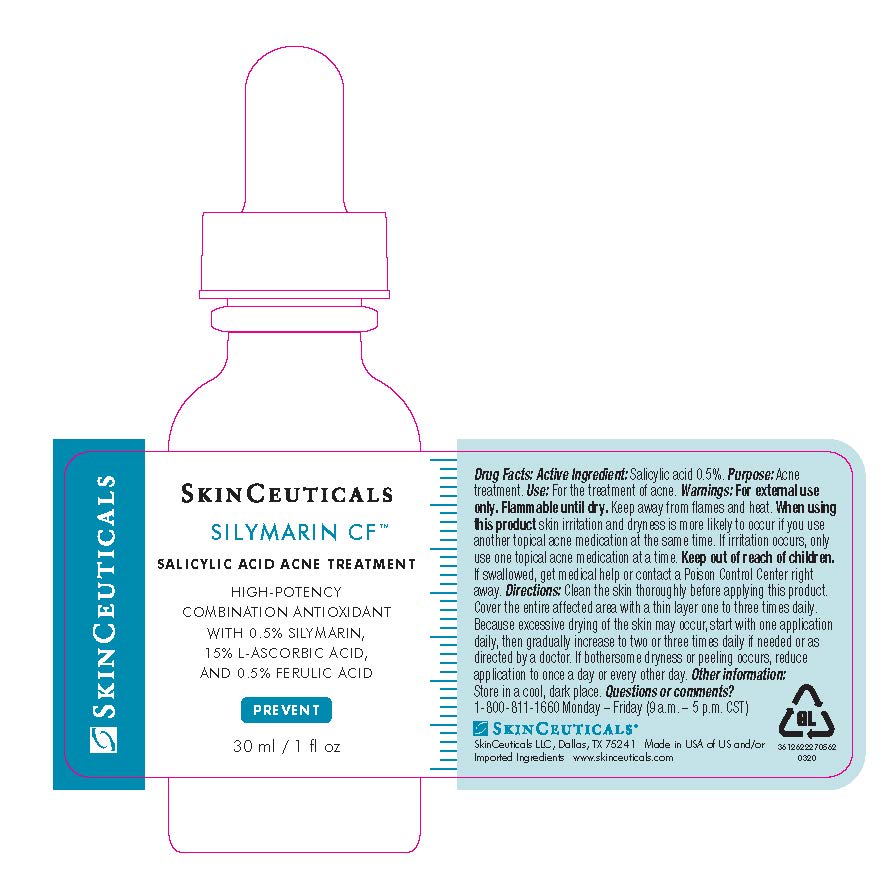
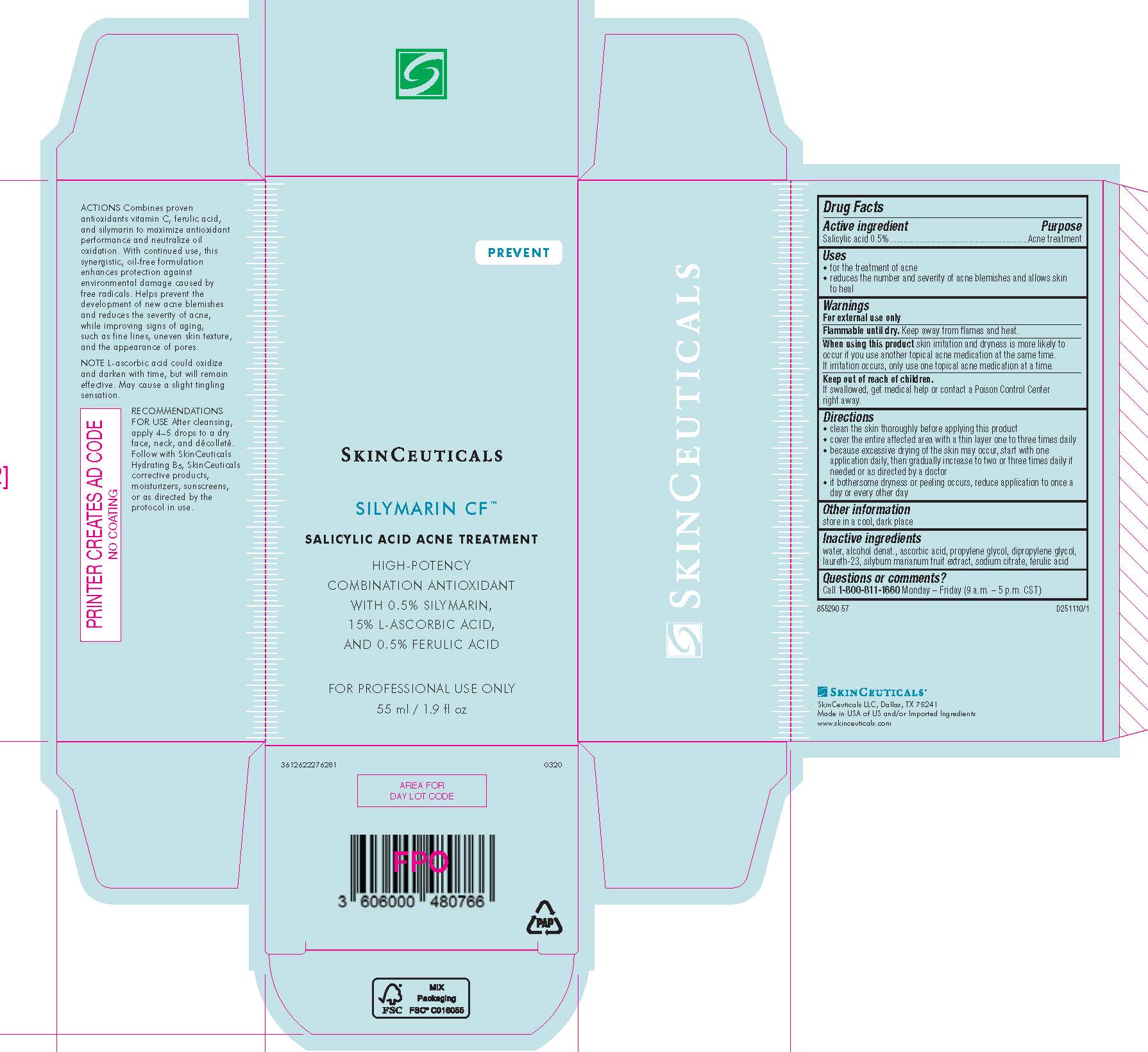
Label: SKINCEUTICALS SILYMARIN CF ACNE TREATMENT- salicylic acid solution
- NDC Code(s): 49967-068-01, 49967-068-02, 49967-068-03, 49967-068-04
- Packager: L'Oreal USA Products Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
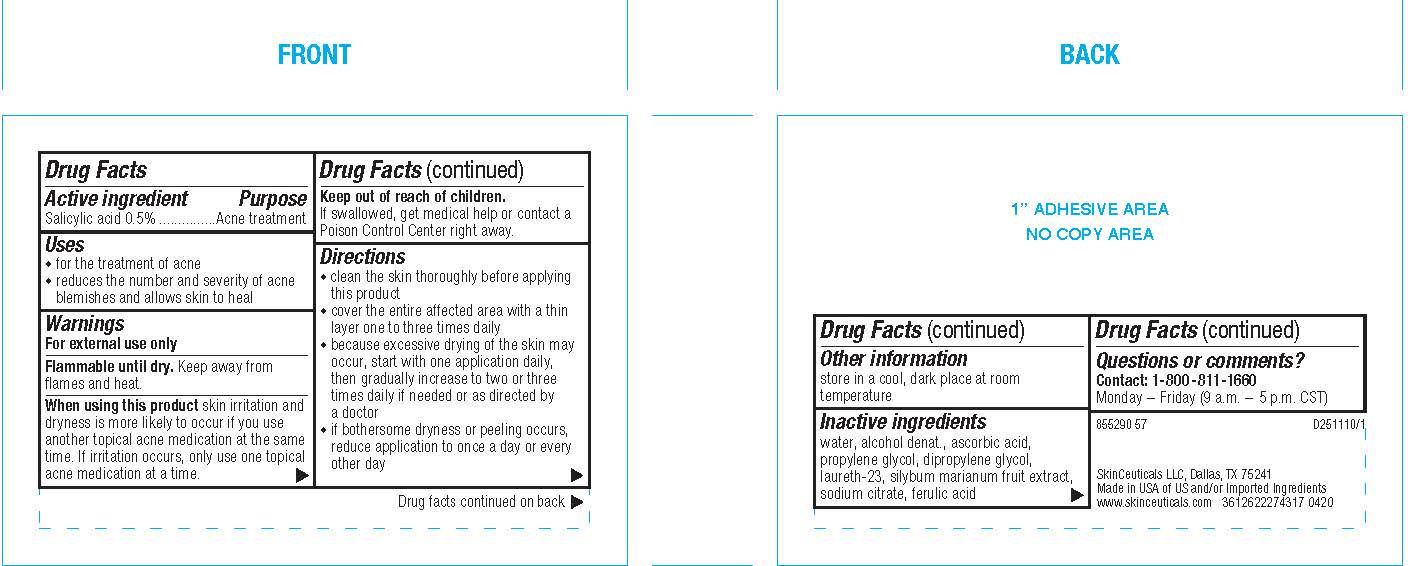
- Active ingredient
- Purpose
- Uses
- Warnings
- Flammable until dry.
- When using this product
- Keep out of reach of children.
-
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- Other information
- Inactive ingredients
- Questions or comments?
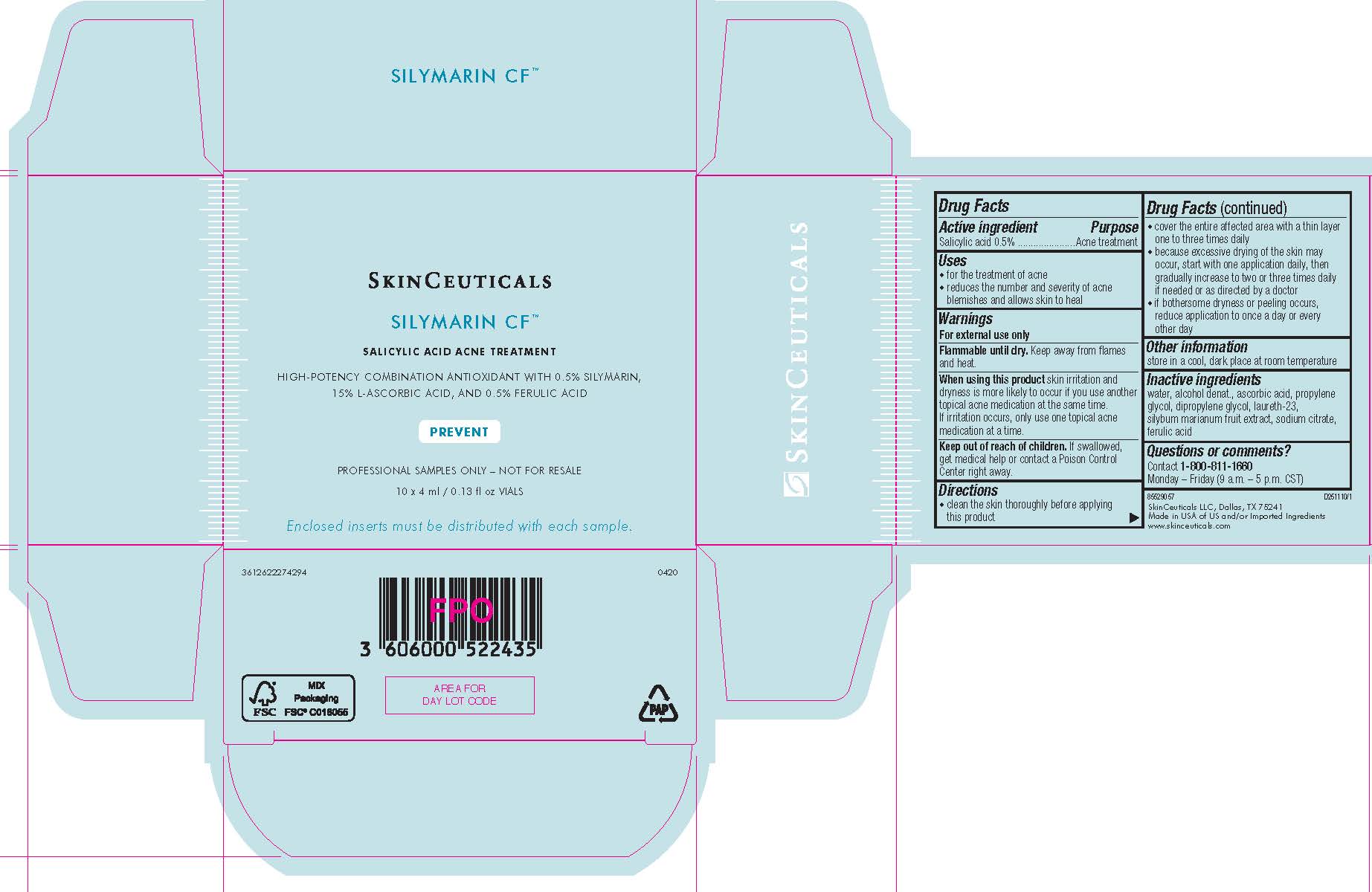
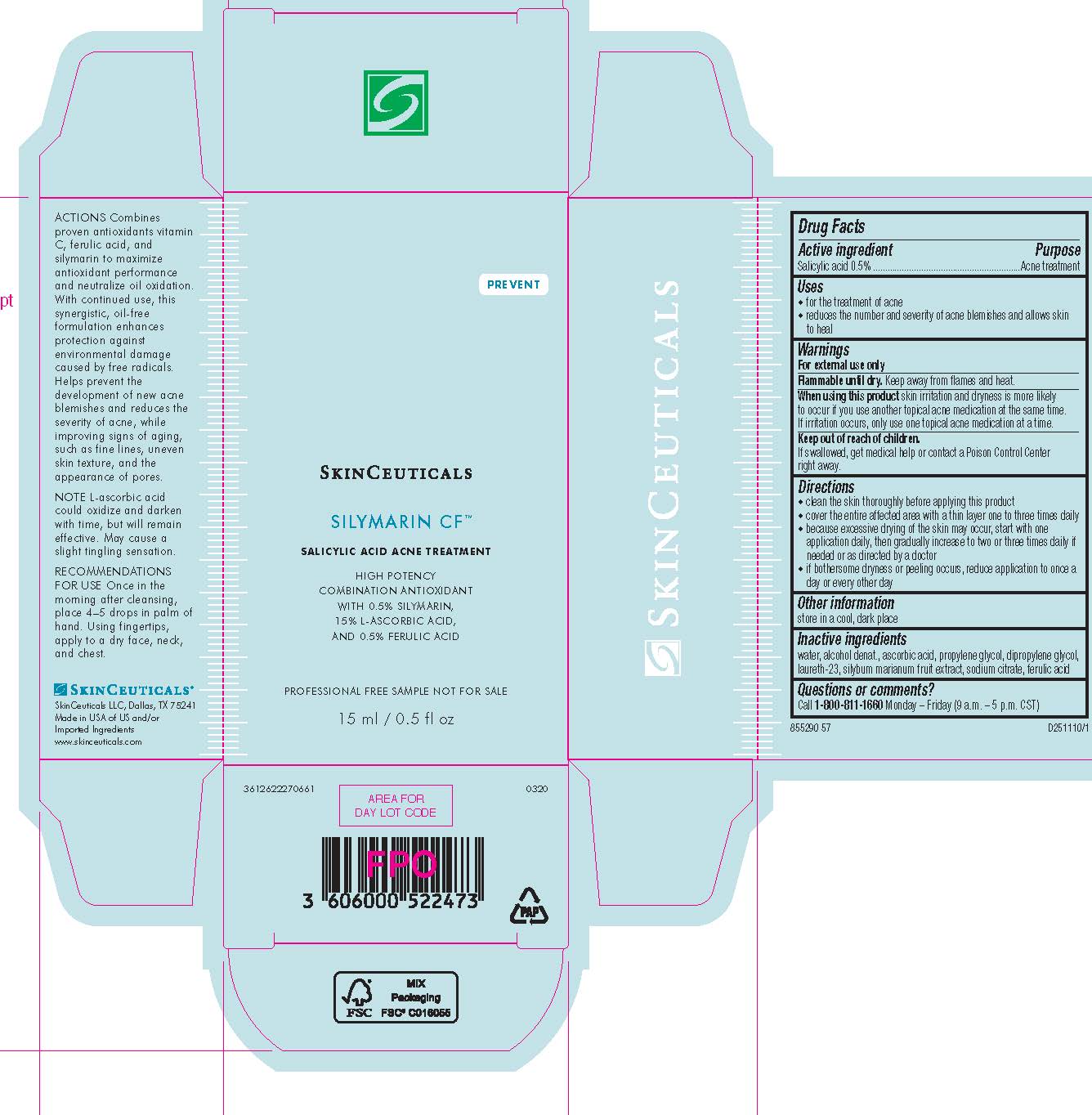
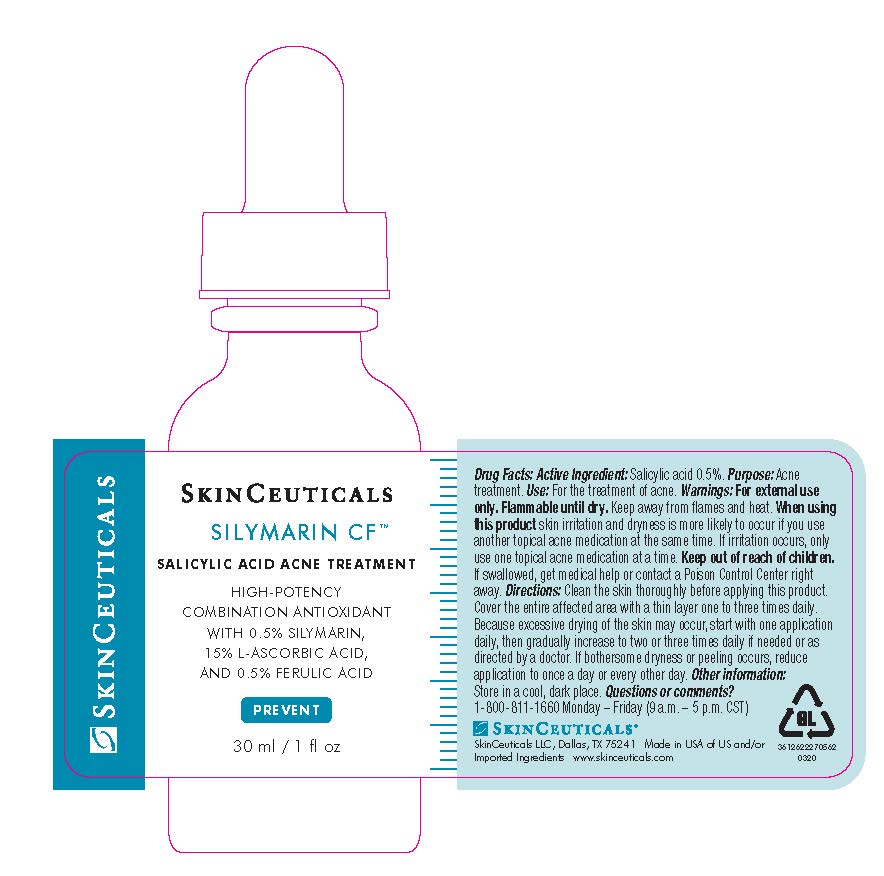
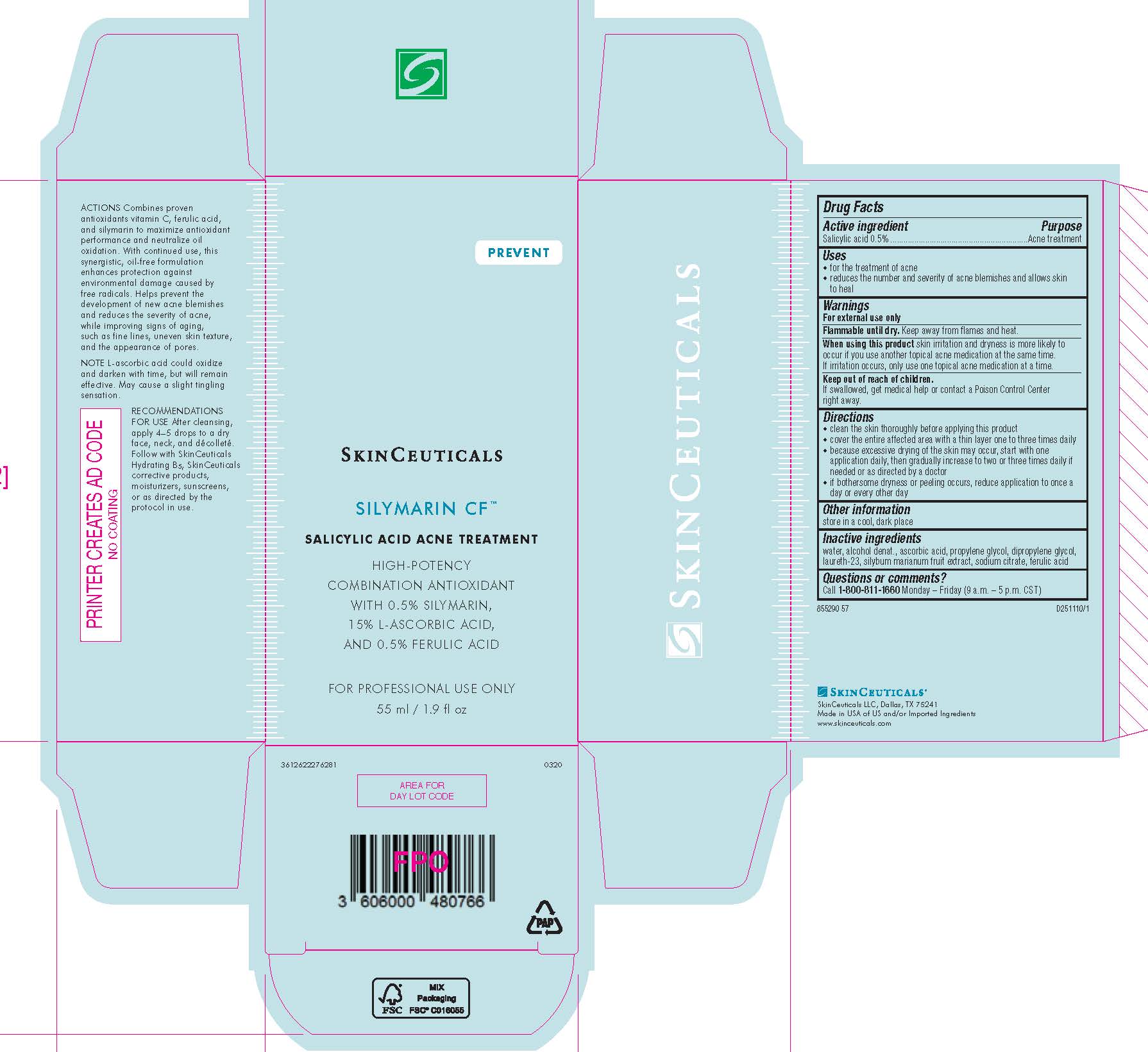
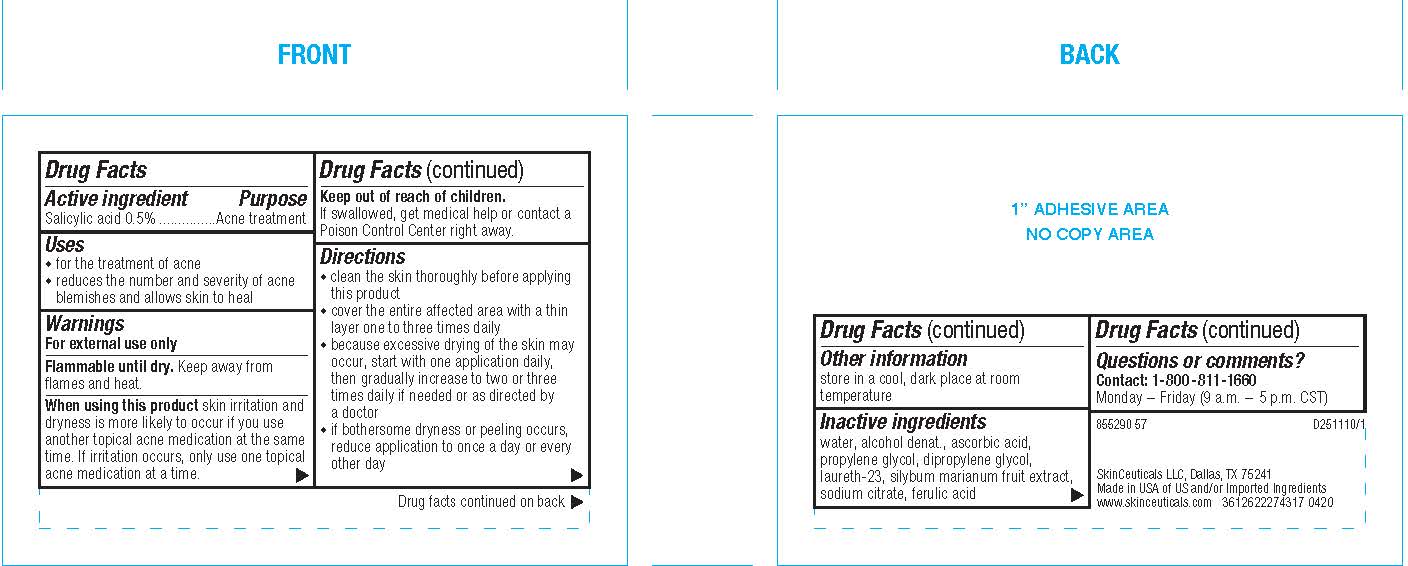
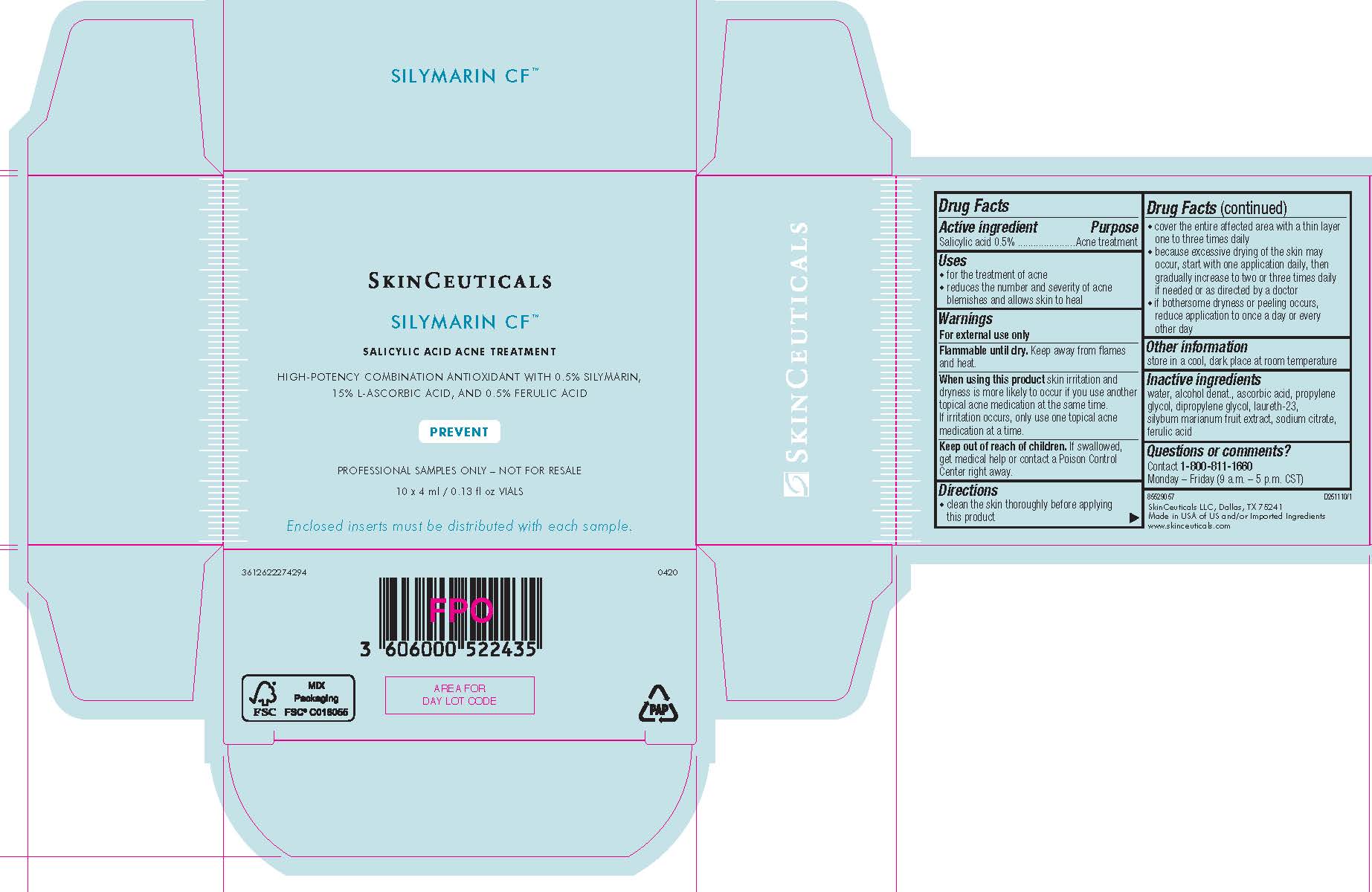
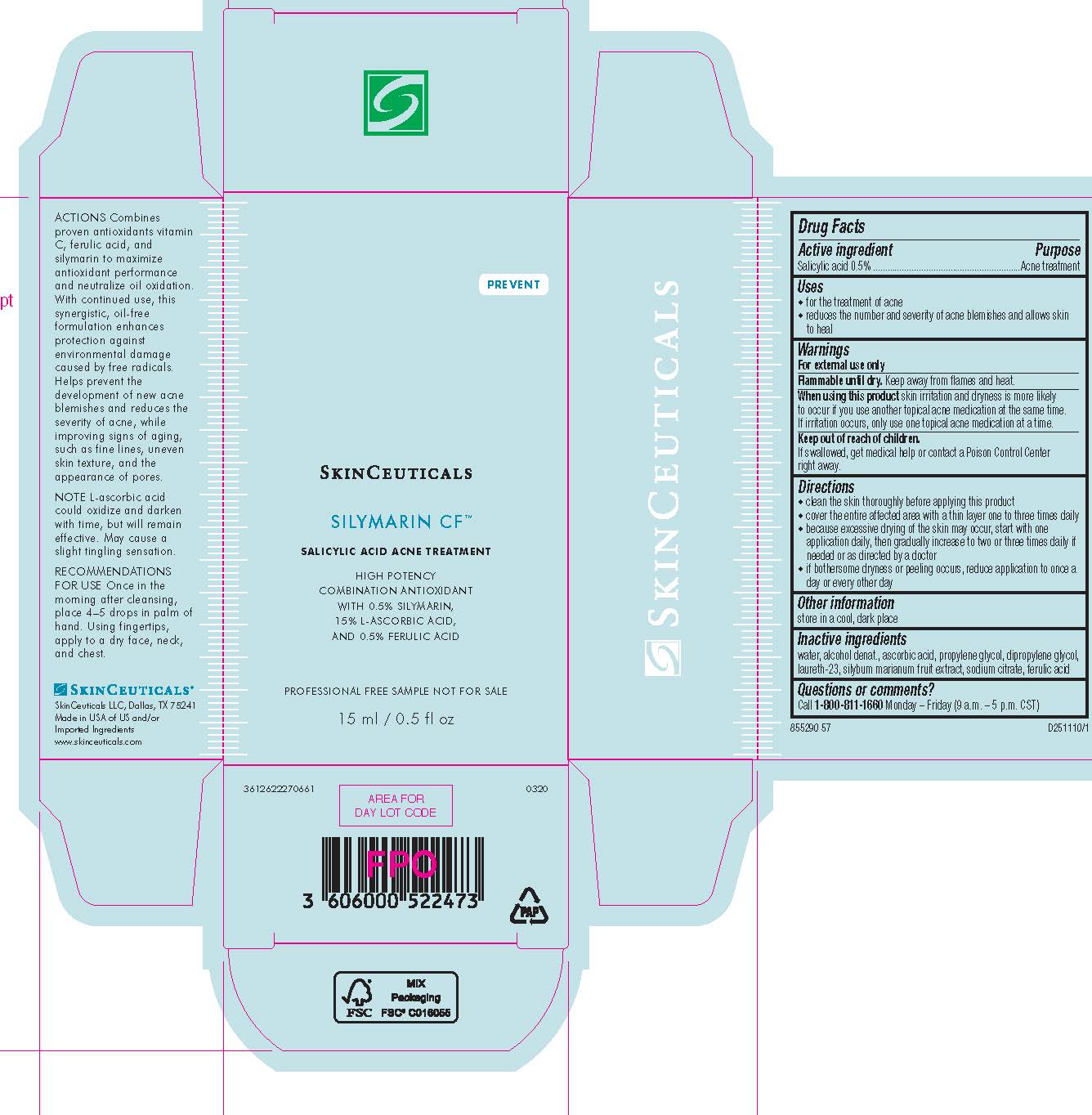
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SKINCEUTICALS SILYMARIN CF ACNE TREATMENT
salicylic acid solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-068 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) ASCORBIC ACID (UNII: PQ6CK8PD0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIPROPYLENE GLYCOL (UNII: E107L85C40) LAURETH-23 (UNII: N72LMW566G) MILK THISTLE (UNII: U946SH95EE) SODIUM CITRATE (UNII: 1Q73Q2JULR) FERULIC ACID (UNII: AVM951ZWST) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-068-01 1 in 1 CARTON 01/01/2021 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:49967-068-02 1 in 1 CARTON 01/01/2021 2 55 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:49967-068-03 1 in 1 CARTON 01/01/2021 3 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:49967-068-04 10 in 1 CARTON 01/01/2021 4 4 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/31/2020 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations Cosmetic Essence, LLC 032565959 manufacture(49967-068) Establishment Name Address ID/FEI Business Operations Cosmetique Active Production 282658798 manufacture(49967-068) Establishment Name Address ID/FEI Business Operations Interspray 364829903 pack(49967-068)