Label: MOCHEASU ALOPECIA-SHAMPOO- zinc pyrithione, salicylic acid shampoo
- NDC Code(s): 79546-201-01
- Packager: Somi T Na Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
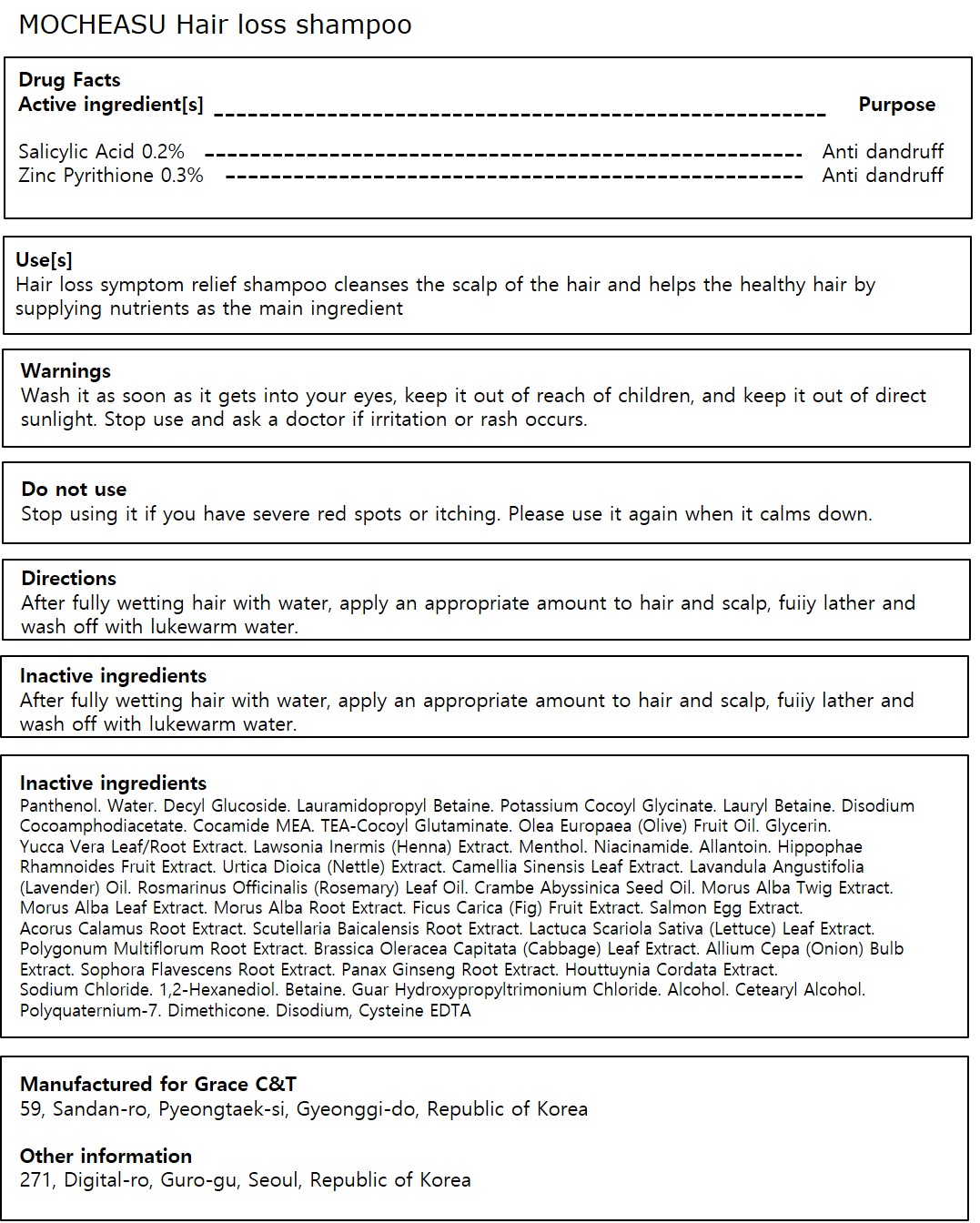
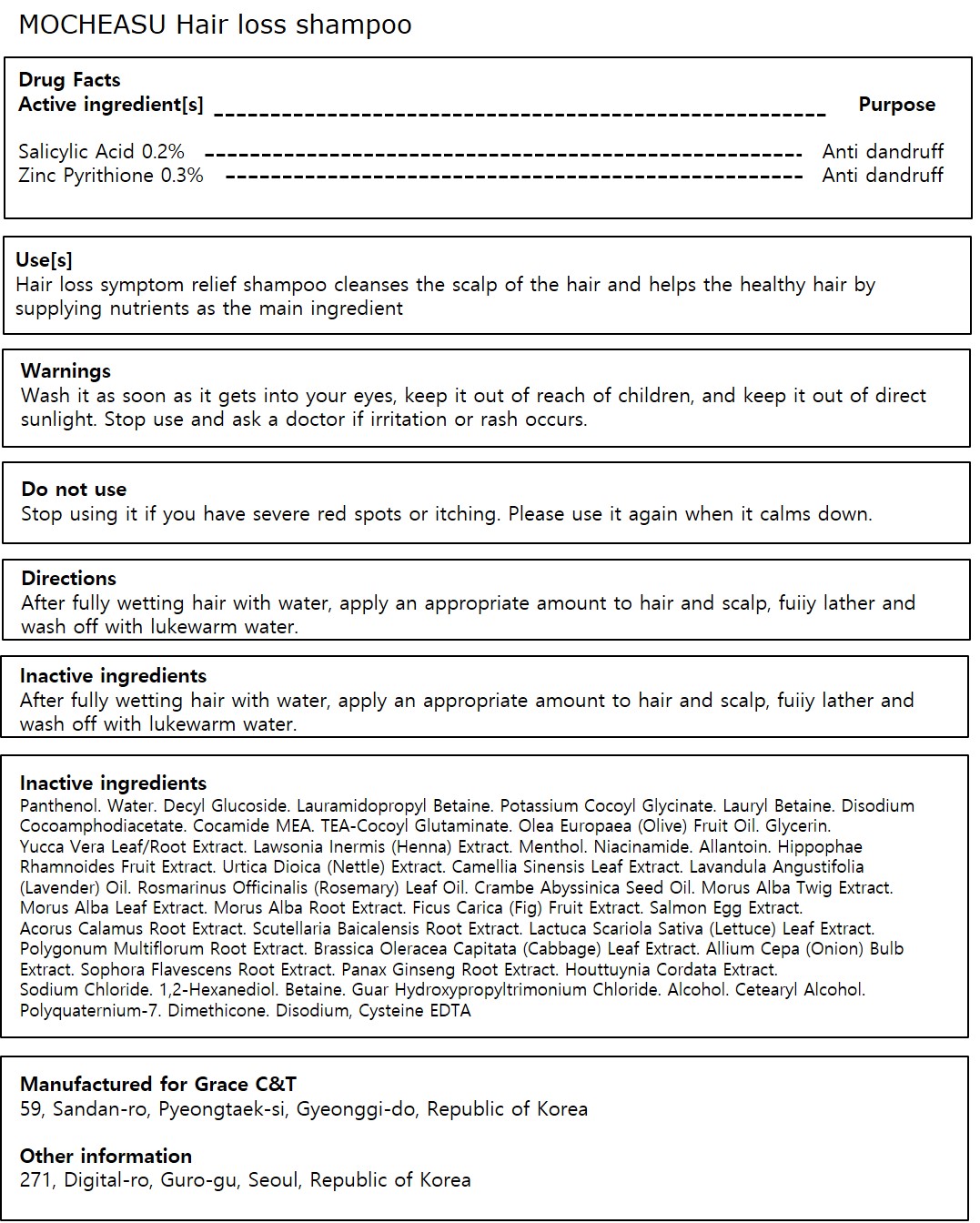
- Active ingredient
- Purpose
- Use
- Warnings
- Warnings
- Warnings
- Warnings
- Directions
-
Inactive ingredients
Water, Decyl Glucoside, Lauramidopropyl Betaine, Potassium Cocoyl Glycinate, Lauryl Betaine, Disodium Cocoamphodiacetate, Cocamide MEA, TEA-Cocoyl Glutaminate, Olea Europaea (Olive) Fruit Oil, Glycerin, Yucca Vera Leaf/Root Extract, Lawsonia Inermis (Henna) Extract, Menthol, Niacinamide, Allantoin, Hippophae Rhamnoides Fruit Extract, Urtica Dioica (Nettle) Extract, Camellia Sinensis Leaf Extract, Lavandula Angustifolia (Lavender) Oil, Rosmarinus Officinalis (Rosemary) Leaf Oil, Crambe Abyssinica Seed Oil, Morus Alba Twig Extract, Morus Alba Leaf Extract, Morus Alba Root Extract, Ficus Carica (Fig) Fruit Extract, Salmon Egg Extract, Acorus Calamus Root Extract, Scutellaria Baicalensis Root Extract, Cysteine, Lactuca Scariola Sativa (Lettuce) Leaf Extract, Polygonum Multiflorum Root Extract, Brassica Oleracea Capitata (Cabbage) Leaf Extract, Allium Cepa (Onion) Bulb Extract, Sophora Flavescens Root Extract, Panax Ginseng Root Extract, Houttuynia Cordata Extract, Sodium Chloride, 1,2-Hexanediol, Betaine, Guar Hydroxypropyltrimonium Chloride, Alcohol, Cetearyl Alcohol, Polyquaternium-7, Dimethicone, Disodium EDTA
- Package Label
-
INGREDIENTS AND APPEARANCE
MOCHEASU ALOPECIA-SHAMPOO
zinc pyrithione, salicylic acid shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79546-201 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 0.3 g in 100 mL SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.2 g in 100 mL Inactive Ingredients Ingredient Name Strength POTASSIUM COCOYL GLYCINATE (UNII: WZ70FUF22U) OLIVE OIL (UNII: 6UYK2W1W1E) PANTHENOL (UNII: WV9CM0O67Z) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) CYSTEINE (UNII: K848JZ4886) SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) ASIAN GINSENG (UNII: CUQ3A77YXI) MORUS ALBA STEM (UNII: 05Y42QCG2T) SALMON, UNSPECIFIED (UNII: 6122W2M0GB) ACORUS CALAMUS ROOT (UNII: XY1K7KIQ0F) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) LETTUCE (UNII: 5PO6NN3RRJ) REYNOUTRIA MULTIFLORA ROOT (UNII: AUZ3VD75MC) ONION (UNII: 492225Q21H) HOUTTUYNIA CORDATA FLOWERING TOP (UNII: RH041UUZ22) SODIUM CHLORIDE (UNII: 451W47IQ8X) BETAINE (UNII: 3SCV180C9W) ALCOHOL (UNII: 3K9958V90M) DIMETHICONE (UNII: 92RU3N3Y1O) LAURYL BETAINE (UNII: Y4P927Q133) TRIETHANOLAMINE COCOYL GLUTAMINATE (UNII: 2R6QKD2Y0M) LAWSONIA INERMIS FLOWERING TOP (UNII: UF2093XE64) MENTHOL (UNII: L7T10EIP3A) NIACINAMIDE (UNII: 25X51I8RD4) ALLANTOIN (UNII: 344S277G0Z) URTICA DIOICA LEAF (UNII: X6M0DRN46Q) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) GUAR HYDROXYPROPYLTRIMONIUM CHLORIDE (1.7 SUBSTITUENTS PER SACCHARIDE) (UNII: B16G315W7A) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYQUATERNIUM-7 (70/30 ACRYLAMIDE/DADMAC; 1600000 MW) (UNII: 0L414VCS5Y) COCO MONOETHANOLAMIDE (UNII: C80684146D) YUCCA SCHIDIGERA (UNII: 08A0YG3VIC) LAURAMIDOPROPYL BETAINE (UNII: 23D6XVI233) DISODIUM COCOAMPHODIACETATE (UNII: 18L9G3U51M) GLYCERIN (UNII: PDC6A3C0OX) GREEN TEA LEAF (UNII: W2ZU1RY8B0) ROSEMARY OIL (UNII: 8LGU7VM393) CABBAGE (UNII: GW0W1Y9I97) WATER (UNII: 059QF0KO0R) HIPPOPHAE RHAMNOIDES FRUIT (UNII: AVL0R9111T) LAVENDER OIL (UNII: ZBP1YXW0H8) CRAMBE HISPANICA SUBSP. ABYSSINICA SEED OIL (UNII: 0QW9S92J3K) MORUS ALBA LEAF (UNII: M8YIA49Q2P) MORUS ALBA ROOT (UNII: CST1G9BZGD) FIG (UNII: TGD87RII2U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79546-201-01 300 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/11/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M032 07/11/2020 Labeler - Somi T Na Co., Ltd (694085583) Registrant - Somi T Na Co., Ltd (694085583) Establishment Name Address ID/FEI Business Operations Somi T Na Co., Ltd 694085583 manufacture(79546-201)