Label: BLACK DECKER HAND SANITIZER GEL- ethyl alcohol gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 74631-006-06, 74631-006-07 - Packager: Dynamic Green Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 29, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
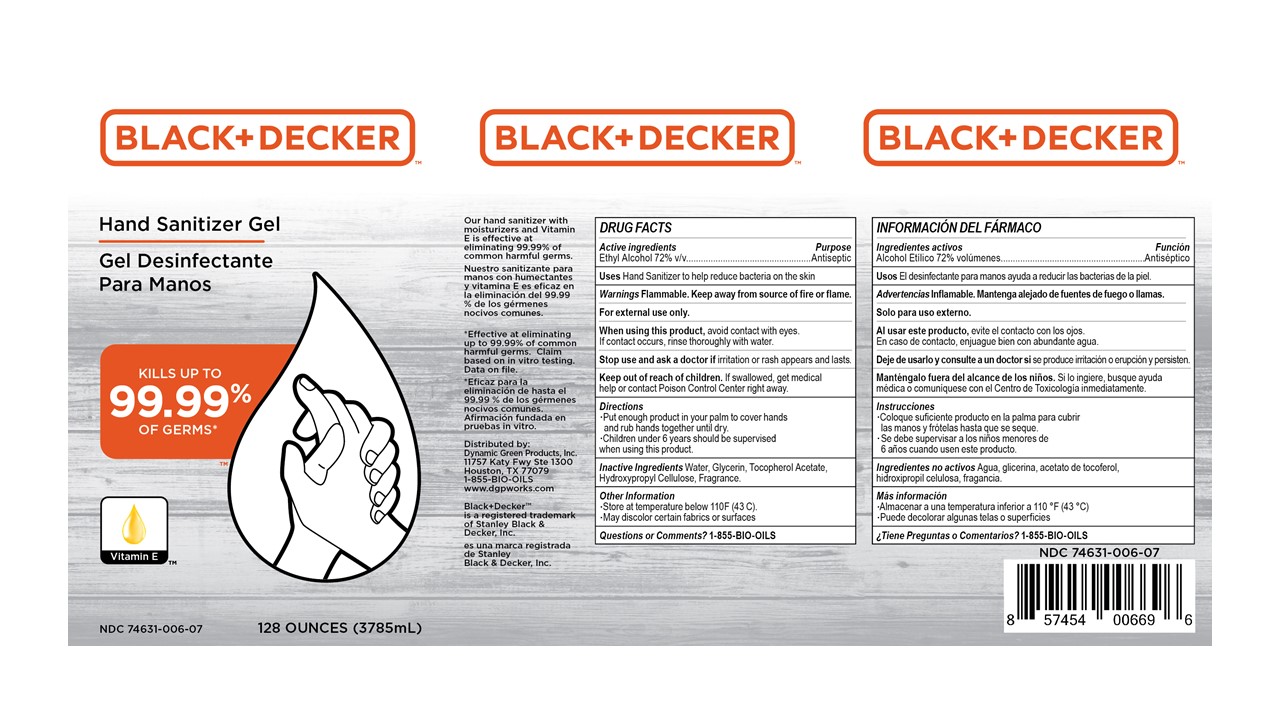
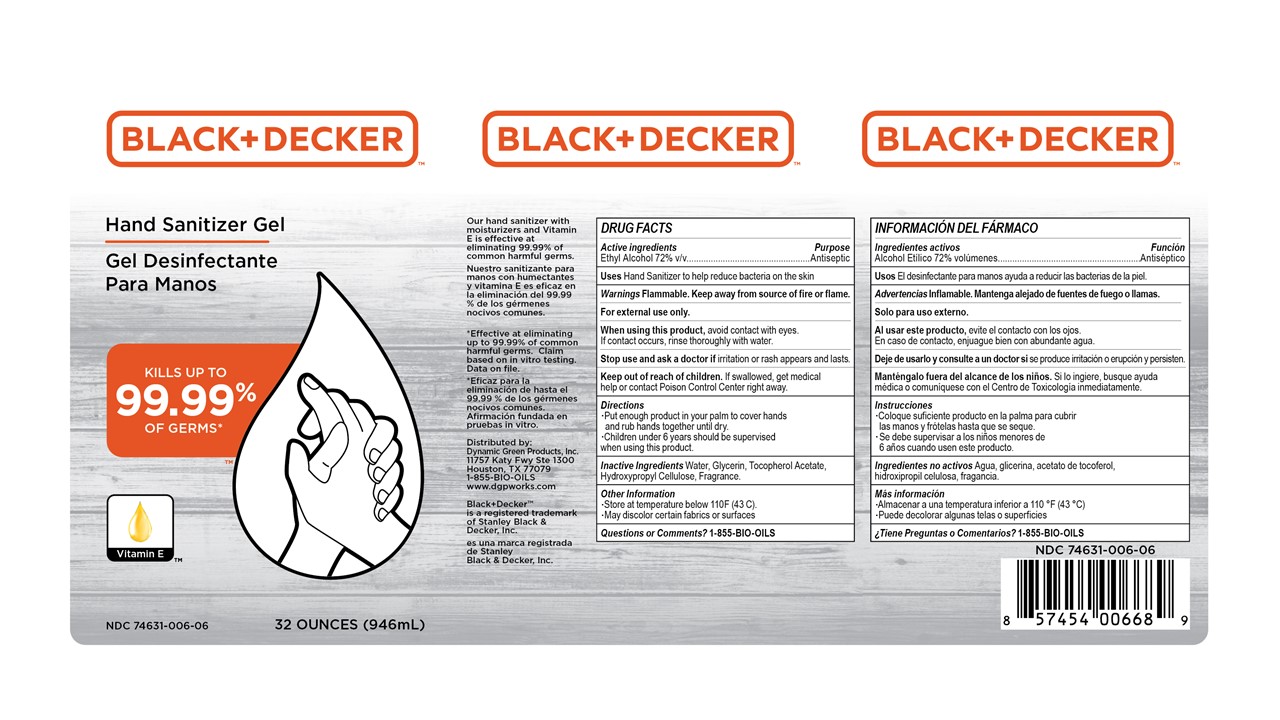
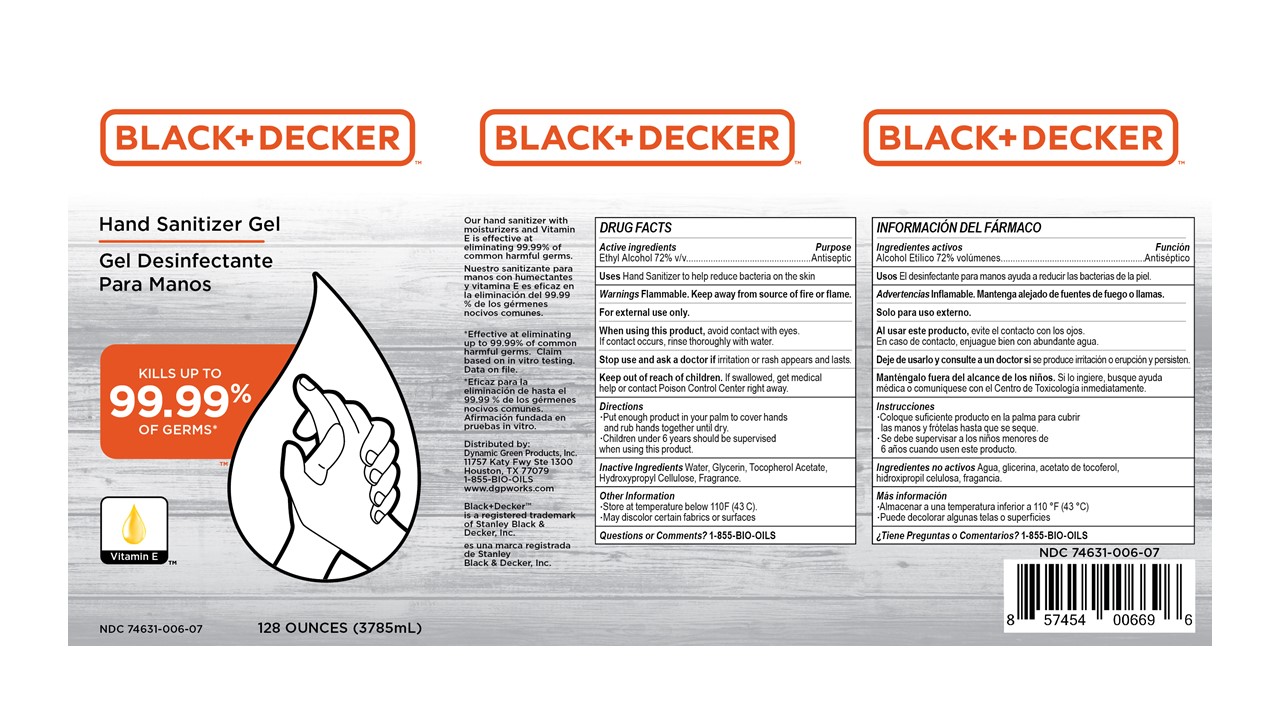
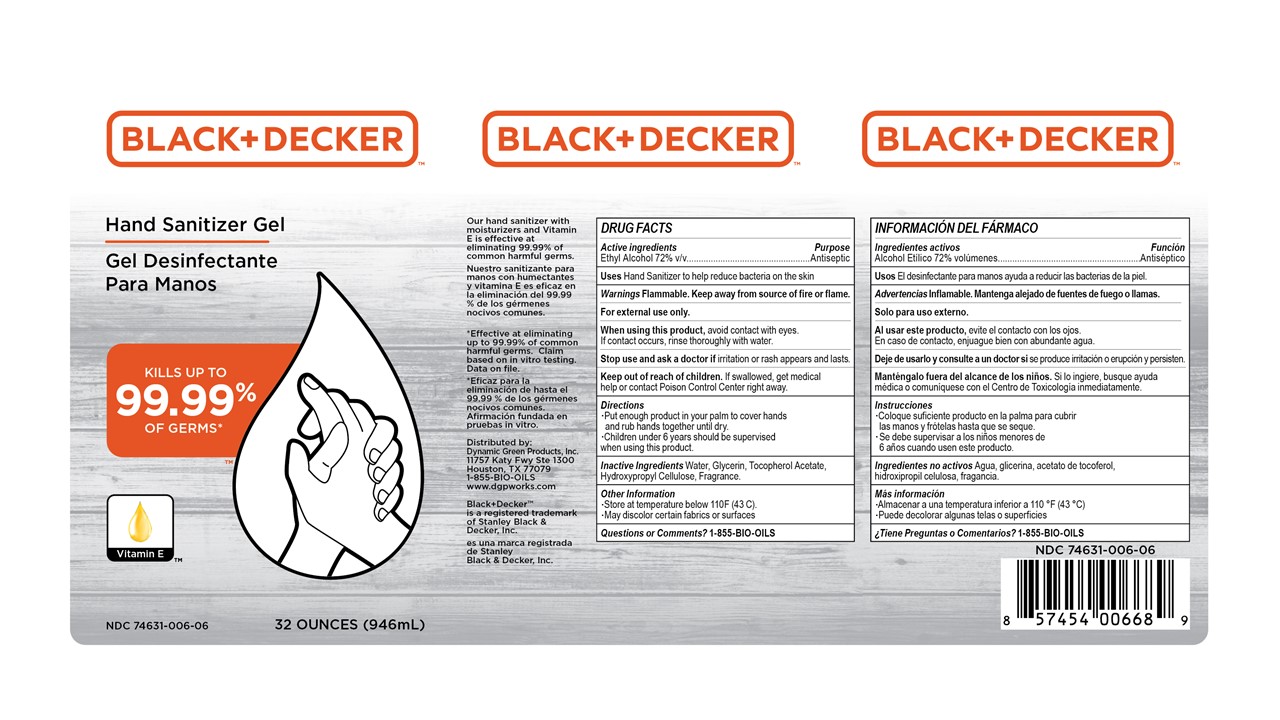
- Active ingredient
- Purpose
- INDICATIONS & USAGE
- WARNINGS
- Directions
- Inactive ingredients
-
Principal Display Panel
Black+Decker Hand Sanitizer Gel
Kills up to 99.99% of Germs
Vitamin E
Our hand sanitizer with moisturizers and Vitamin E is effective at eliminating 99.99% of common harmful germs.
*Effective at eliminating up to 99.99% of commmon harmful germs. Claim based on in vitro testing. Data on file.
Distributed by:
Dynamic Green Products, Inc.
11757 Katy Fwy Ste 1300
Houston, TX 77079
1-855-BIO-OILS
www.dgpworks.com
Black_Decker is a registered trademark of Stanley Black & Decker, Inc.
XX oz (zzzmL) NDC 74631-006-xx
-
INGREDIENTS AND APPEARANCE
BLACK DECKER HAND SANITIZER GEL
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74631-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 72 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GRAPEFRUIT OIL (UNII: YR377U58W9) HYDROXYPROPYL CELLULOSE (110000 WAMW) (UNII: 5Y0974F5PW) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74631-006-07 3785 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/08/2020 2 NDC:74631-006-06 946 mL in 1 BOTTLE; Type 0: Not a Combination Product 07/08/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 07/08/2020 Labeler - Dynamic Green Products, Inc. (110949053) Registrant - Dynamic Green Products, Inc. (110949053) Establishment Name Address ID/FEI Business Operations Packaging Service Co 008435950 repack(74631-006) , pack(74631-006) Establishment Name Address ID/FEI Business Operations Vertrauen Chemie Solutions Inc 080251121 pack(74631-006) , repack(74631-006) Establishment Name Address ID/FEI Business Operations BPI Coatings Solutions LLC 080477067 pack(74631-006) , repack(74631-006) Establishment Name Address ID/FEI Business Operations BRENNTAG MID-SOUTH INC 122625064 analysis(74631-006) , manufacture(74631-006)