Label: SENNA- sennosides syrup
SENNA UD- sennosides syrup
-
NDC Code(s):
39328-020-08,
39328-120-05,
39328-120-50,
39328-120-99, view more39328-220-15, 39328-220-50
- Packager: Patrin Pharma, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
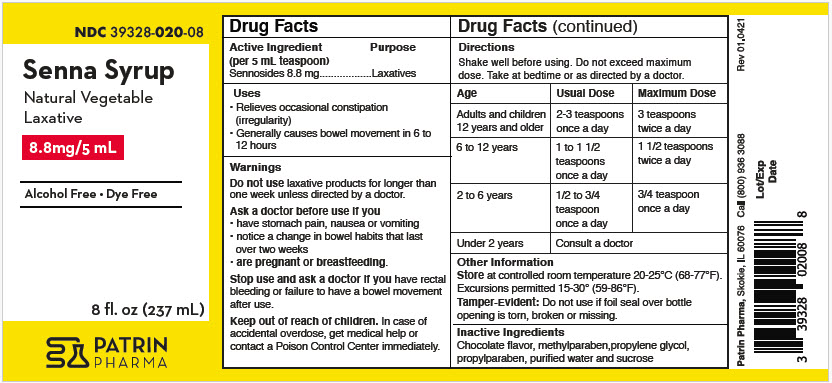
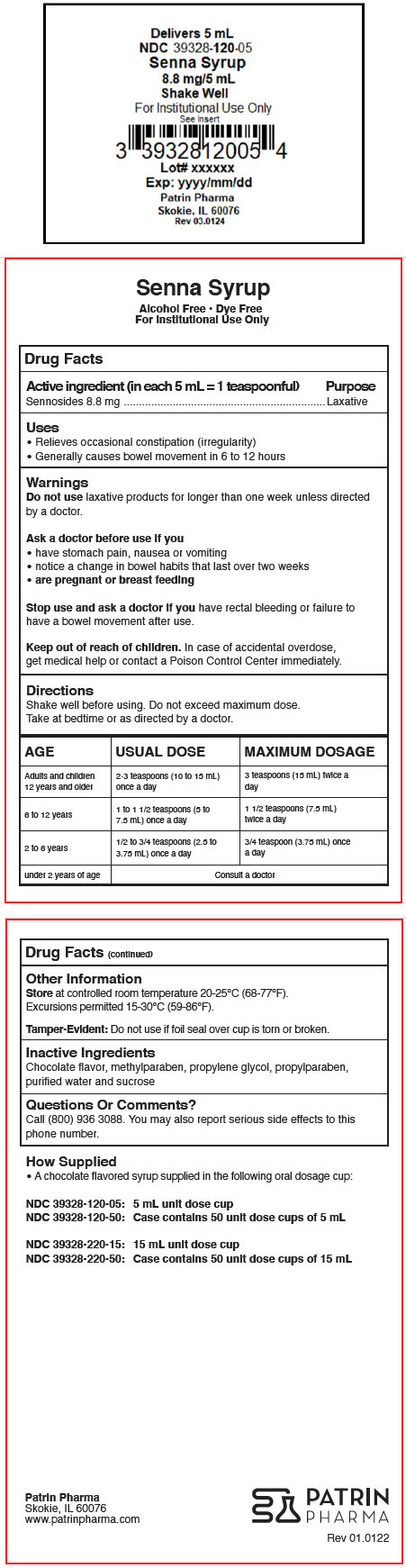
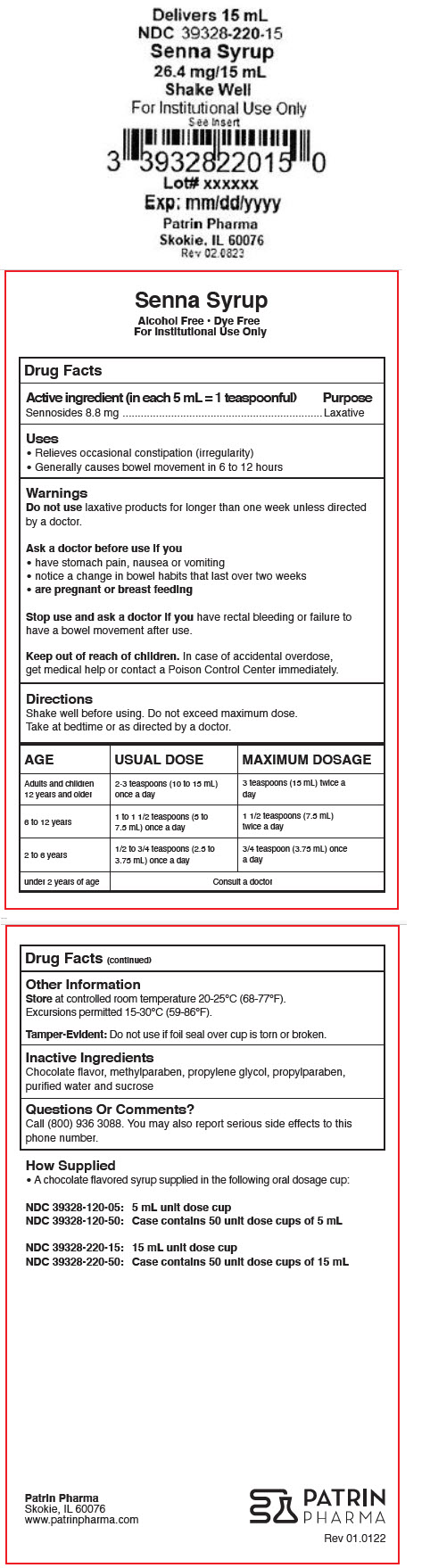
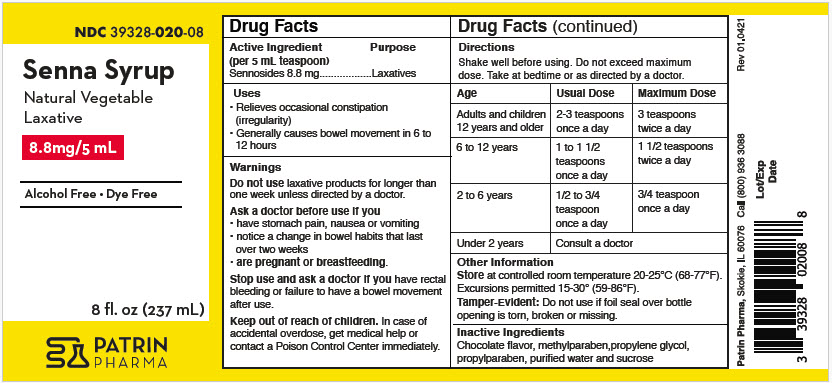
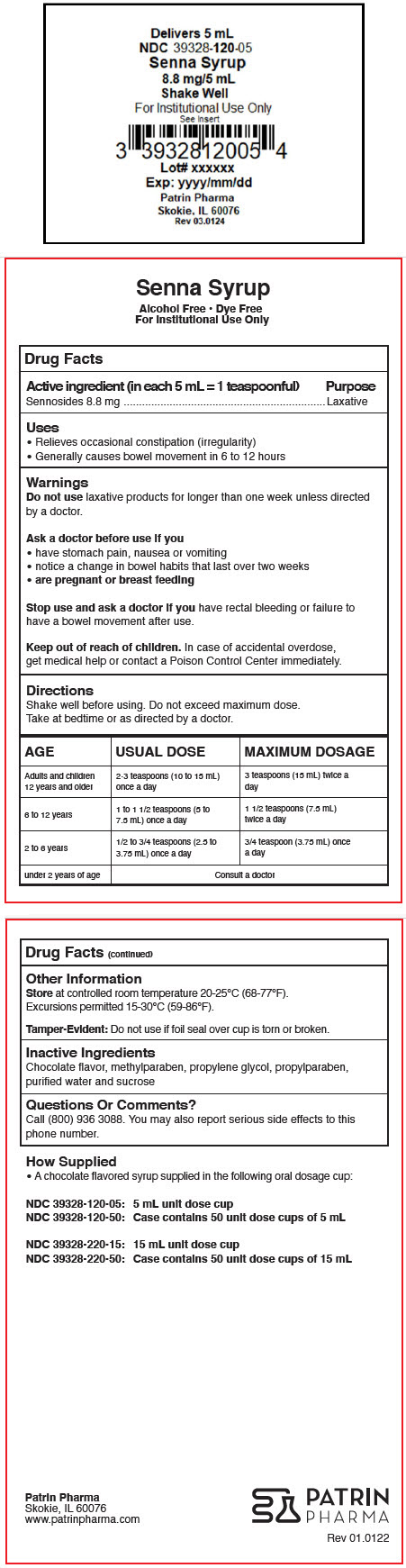
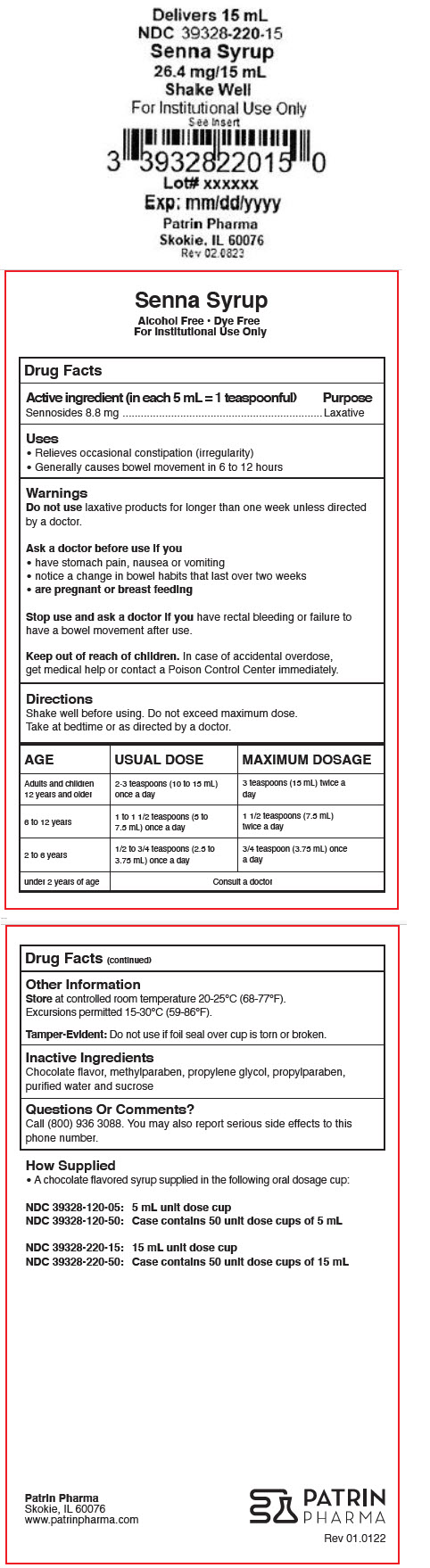
- Active ingredient (in each 5 mL = 1 teaspoonful)
- Purpose
- Uses
- Warnings
-
Directions
Shake well before using. Do not exceed maximum dose.
Take at bedtime or as directed by a doctor.
AGE USUAL DOSE MAXIMUM DOSAGE Adults and children 12 years and older 2-3 teaspoons (10 to 15 mL) once a day 3 teaspoons (15 mL) twice a day 6 to 12 years 1 to 1 1/2 teaspoons (5 to 7.5 mL) once a day 1 1/2 teaspoons (7.5 mL) twice a day 2 to 6 years 1/2 to 3/4 teaspoons (2.5 to 3.75 mL) once a day 3/4 teaspoon (3.75 mL) once a day under 2 years of age Consult a doctor - Other Information
- Inactive Ingredients
- Questions Or Comments?
- How Supplied
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 237 mL Bottle Label
- PRINCIPAL DISPLAY PANEL - 5 mL Cup Label
- PRINCIPAL DISPLAY PANEL - 15 mL Cup Label
-
INGREDIENTS AND APPEARANCE
SENNA
sennosides syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:39328-020 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sennosides (UNII: 3FYP5M0IJX) (Sennosides - UNII:3FYP5M0IJX) Sennosides 8.8 mg in 5 mL Inactive Ingredients Ingredient Name Strength methylparaben (UNII: A2I8C7HI9T) propylene glycol (UNII: 6DC9Q167V3) propylparaben (UNII: Z8IX2SC1OH) water (UNII: 059QF0KO0R) sucrose (UNII: C151H8M554) Product Characteristics Color BROWN Score Shape Size Flavor CHOCOLATE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:39328-020-08 237 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M007 10/01/2023 SENNA UD
sennosides syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:39328-120 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sennosides (UNII: 3FYP5M0IJX) (Sennosides - UNII:3FYP5M0IJX) Sennosides 8.8 mg in 5 mL Inactive Ingredients Ingredient Name Strength methylparaben (UNII: A2I8C7HI9T) propylene glycol (UNII: 6DC9Q167V3) propylparaben (UNII: Z8IX2SC1OH) water (UNII: 059QF0KO0R) sucrose (UNII: C151H8M554) Product Characteristics Color BROWN Score Shape Size Flavor CHOCOLATE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:39328-120-50 5 in 1 CASE 10/01/2023 1 10 in 1 TRAY 1 NDC:39328-120-05 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product 2 NDC:39328-120-99 10 in 1 CASE 06/03/2024 2 10 in 1 TRAY 2 NDC:39328-120-05 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M007 10/01/2023 SENNA UD
sennosides syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:39328-220 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sennosides (UNII: 3FYP5M0IJX) (Sennosides - UNII:3FYP5M0IJX) Sennosides 26.4 mg in 15 mL Inactive Ingredients Ingredient Name Strength methylparaben (UNII: A2I8C7HI9T) propylene glycol (UNII: 6DC9Q167V3) propylparaben (UNII: Z8IX2SC1OH) water (UNII: 059QF0KO0R) sucrose (UNII: C151H8M554) Product Characteristics Color BROWN Score Shape Size Flavor CHOCOLATE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:39328-220-50 5 in 1 CASE 10/01/2023 1 10 in 1 TRAY 1 NDC:39328-220-15 15 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M007 10/01/2023 Labeler - Patrin Pharma, Inc. (806841677)