Label: TERUFLEX BLOOD BAG SYSTEM ANTICOAGULANT CITRATE PHOSPHATE DEXTROSE (CPD) AND OPTISOL RED CELL PRESERVATIVE (anticoagulant citrate phosphate dextrose- cpd and as-5 red cell preservative kit
- NDC Code(s): 53877-005-01
- Packager: Terumo Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 5, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
INDICATIONS & USAGE
TERUFLEX® BLOOD BAG SYSTEM
CPD/OPTISOL® SOLUTION BLOOD BAG SYSTEM
Read these instructions carefully before use. Rx ONLY.
INTENDED USE
Collection of 250 mL Whole Blood:
• From elderly, pediatric, or smaller autologous donors who may not tolerate larger blood collections.
• For blood collections as directed by a patient's physician.
• Platelets prepared from 250 mL whole blood collections can only be used for the autologous donation patient.
• Platelet preparation from a 250 mL whole blood collection are unlikely to meet the 5.5x1010 platelet concentration required by the regulations.
INSTRUCTIONS FOR BLOOD COLLECTION: Use aseptic technique
1. Confirm that all numbered tubing of each blood bag unit contains its own identical segment numbers.
2. Make a loose knot in the donor tubing approximately 10 cm from the needle.
3. Clamp donor tubing with hemastat.
4. Suspend primary bag a minimum of 60 cm below the donor's arm
5. Apply blood pressure cuff. Disinfect site of phlebotomy. Inflate blood pressure cuff to 60 mmHg.
6. Remove needle protector and perform phlebotomy. Remove hemastat to permit blood flow into primary bag.
CAUTION Do not touch needle after removing the needle protector.
7. Tape donor tubing securely to donor's arm.
8. MIX BLOOD WITH ANTICOAGULANT AT SEVERAL INTERVALS DURING COLLECTION.
9. Collect 250 mL of blood.
10. Tighten knot firmly after collection. Clamp between knot and needle. Sever donor tubing between knot and clamp. Collect pilot samples.
11. Reapply clamp to donor tubing; release blood pressure cuff and remove needle from donor's arm.
CAUTION Discard tubing/phlebotomy needle unit according to institutional procedures.
12. Immediately after collection, invert bag several times mixing blood with anticoagulant thoroughly.
13. Strip blood from donor tubing into bag, mix well, and allow tubing to refill. Seal at X marks on donor tubing to provide numbered aliquots of anticoagulated blood for testing.
CAUTION Begin sealing at needle end and work towards bag.
14.The time of addition of OPTISOL solution may vary depending on the processing option selected. Add solution under one of the following conditions.
a) After removal of plasma from freshly collected blood.
b) Within 8 hours of blood collection if platelets are prepared.
c) Within 72 hours of collection if blood is refrigerated immediately following collection.
15. Centrifuge unit to separate red cells from plasma.
16. Snap CLIKTIP (incline closure device) of primary collection bag and transfer plasma into satellite bag. Clamp transfer tubing of satellite bag.
17. Snap CLIKTIP of OPTISOL solution bag and drain contents into primary bag containing red blood cells. Seal tubing of primary bag in two places, cut between seals, and separate from satellite bag.
NOTE: For TERUFLEX double bags, seal OPTISOL solution bag tubing in two places, but between seals, and separate. Discard OPTISOL solution container.
18. Mix OPTISOL solution and red cells thoroughly. Store between 1-6oC.
19. Infuse OPTISOL red blood cells within 42 days of collection.
For further processing, use standard component processing techniques.
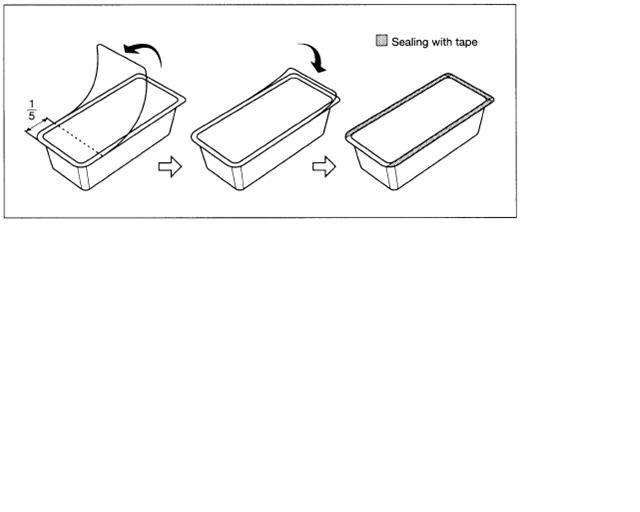
To open the blister package, peel the cover film back 4/5 of its length. (See Fig. 3)
After opening, unused blood bags may be stored at room temperature for 96 hours, or they may be stored for 30 days in the blister package after returning the cover film to the original position and sealing with tape to prevent possible loss of moisture.
CAUTIONS
• THE PACKET OF AGELESS CONTAINED IN THIS PACKAGE GENERATES HEAT UPON REMOVAL AND SHOULD BE HANDLED WITH CARE.
• DISPOSE WITH AGELESS PACKET IN TRAY.
• DO NOT DISPOSE WITH WASTES CONTAINING VOLATILE OR FLAMMABLE MATERIALS.
• DISCARD AGELESS PACKET WITHOUT OPENING.
DO NOT USE UNLESS ANTICOAGULANT IS CLEAR.
RECOMMENDED STORAGE: Room Temperature (25oC/77oF).
Avoid excessive heat and direct sunlight. Protect from freezing.
TERUMO CORPORATION
44-1, 2-CHOME, HATAGAYA, SHIBUYA-KU, TOKYO, JAPAN
TERUMO and TERUFLX are registered in U.S. Patent and Trademark Office
by TERUMO CORPORATION.
OPTISOL and CLIKTIP are trademarks of TERUMO CORPORATION.
Issued 2/95
N-BB-OP-A3
-
PRINCIPAL DISPLAY PANEL
Tray/Case Label
TERUFLEX® BLOOD BAG SYSTEM with Blood Sampling Arm®
CPD WITH OPTISOL® RED CELL PRESERVATIVE SOLUTION
FOR COLLECTION OF 250 mL OF BLOOD
Each unit consists of a primary bag containing 35mL of Anticoagulant
CPD solution, with a satellite bag containing 56mL of OPTISOL Red
Cell Preservative Solution.
Each 35 mL Anticoagulant CPD solution USP contains 893mg Dextrose
(monohydrate) USP, 921mg Sodium Citrate (dihydrate) USP,105mg Citric
Acid (anhydrous) USP, 77.7mg Monobasic Sodium Phosphate
(monohydrate) USP.
Each 56 mL OPTISOL Red Cell Preservative Solution contains 491mg
Sodium Chloride USP, 504mg Dextrose (monohydrate) USP, 294mg
Mannitol USP, 16,8mg Adenine USP.
STERILE, NON-PYROGENIC FLUID PATH.
DO NOT USE UNLESS ANTICOAGULANT IS CLEAR
CODE
LOT No.
EXPIRY
UNITS
DONOR NEEDLE 17G x 1 1/2˝ (1.40 x 38mm)
Rx ONLY
RECOMMENDED STORAGE: Room Temperature (15-30°C/59-86°F).
Avoid excessive heat. Protect from freezing.
After opening, unused bags may be stored for 30 days by returning cover film to original position and
sealing with tape to prevent possible loss of moisture. See Instructions For Blood Collection.
Manufactured by : TERUMO CORPORATION Tokyo, Japan
® : Registered Trademark of TERUMO CORPORATION
Rev. 07/04
B-4-B7-A4 2

-
INGREDIENTS AND APPEARANCE
TERUFLEX BLOOD BAG SYSTEM ANTICOAGULANT CITRATE PHOSPHATE DEXTROSE (CPD) AND OPTISOL RED CELL PRESERVATIVE
anticoagulant citrate phosphate dextrose (cpd) and as-5 red cell preservative kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:53877-005 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53877-005-01 30 in 1 CASE 1 1 in 1 BAG; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BAG 35 mL Part 2 1 BAG 56 mL Part 1 of 2 ANTICOAGULANT CITRATE PHOSPHATE DEXTROSE (CPD)
anticoagulant citrate phosphate dextrose (cpd) solutionProduct Information Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Trisodium Citrate Dihydrate (UNII: B22547B95K) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 26.3 g in 1000 mL SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) (PHOSPHATE ION - UNII:NK08V8K8HR, SODIUM CATION - UNII:LYR4M0NH37) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM 2.22 g in 1000 mL Dextrose Monohydrate (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) Dextrose Monohydrate 25.5 g in 1000 mL Anhydrous Citric Acid (UNII: XF417D3PSL) (Anhydrous Citric Acid - UNII:XF417D3PSL) Anhydrous Citric Acid 2.99 g in 1000 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 35 mL in 1 BAG; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN880217 12/15/2009 Part 2 of 2 OPTISOL RED CELL PRESERVATIVE
as-5 red cell preservative solutionProduct Information Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Chloride (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) Sodium Chloride 877 mg in 100 mL Dextrose Monohydrate (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) Dextrose Monohydrate 900 mg in 100 mL Mannitol (UNII: 3OWL53L36A) (Mannitol - UNII:3OWL53L36A) Mannitol 525 mg in 100 mL Adenine (UNII: JAC85A2161) (Adenine - UNII:JAC85A2161) Adenine 30 mg in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 56 mL in 1 BAG; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN880217 12/15/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN880217 12/15/2009 Labeler - Terumo Corporation (690543319) Establishment Name Address ID/FEI Business Operations Terumo Corp. - Fujinomiya Factory 695214015 manufacture(53877-005) , STERILIZE(53877-005) , ANALYSIS(53877-005) , LABEL(53877-005)

