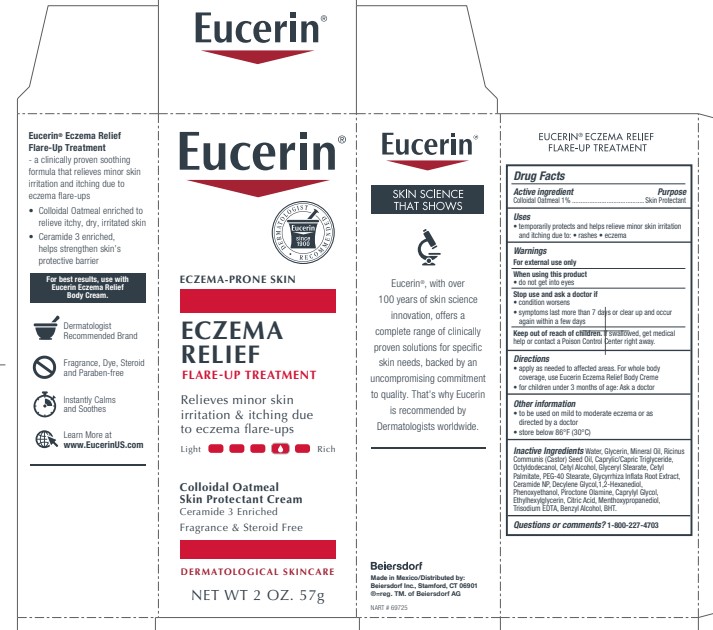
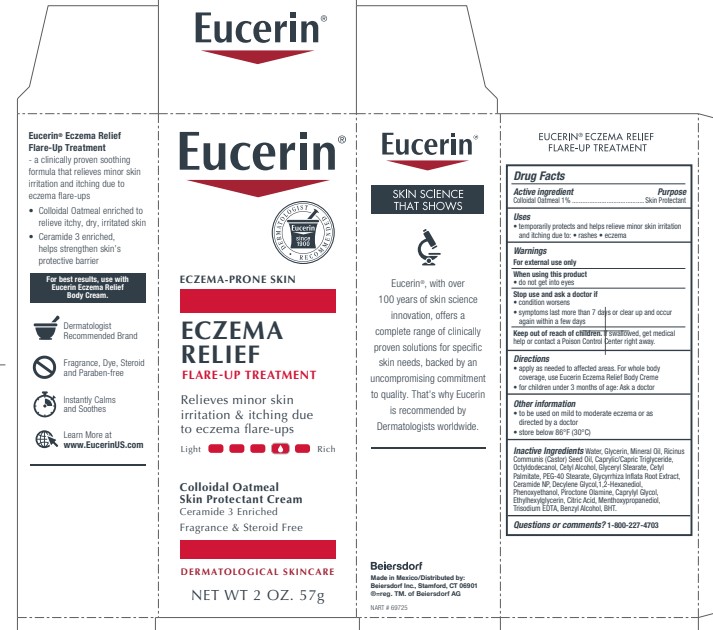
Label: EUCERIN ECZEMA RELIEF FLARE UP TREATMENT- oatmeal cream
- NDC Code(s): 10356-371-07, 10356-371-25, 10356-371-35
- Packager: Beiersdorf Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- WARNINGS
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- QUESTIONS
-
INACTIVE INGREDIENT
Inactive Ingredients
Water, Glycerin, Mineral Oil, Ricinus Communis (Castor) Seed Oil,
Caprylic/Capric Triglyceride, Octyldodecanol, Cetyl Alcohol, Glyceryl Stearate,
Cetyl Palmitate, PEG-40 Stearate, Glycyrrhiza Inflata
Root Extract, Ceramide 3, Decylene Glycol, 1,2-Hexanediol, Phenoxyethanol, Piroctone
Olamine, Caprylyl Glycol, Ethylhexylglycerin, Citric Acid, Menthoxypropanediol,
Trisodium EDTA, Benzyl Alcohol, BHT.
. - PURPOSE
-
PRINCIPAL DISPLAY PANEL
Eucerin
Eczema Relief Flare-Up Treatment
Eczema-Prone Skin
Colloidal Oatmeal Skin Protectant Cream
Ceramide 3 Enriched
Relieves minor skin irritation and
itching due to eczema
flare-upsFragrance Free and Steroid Free
Dermatological skincare
Eucerin Dermatologist Recommended
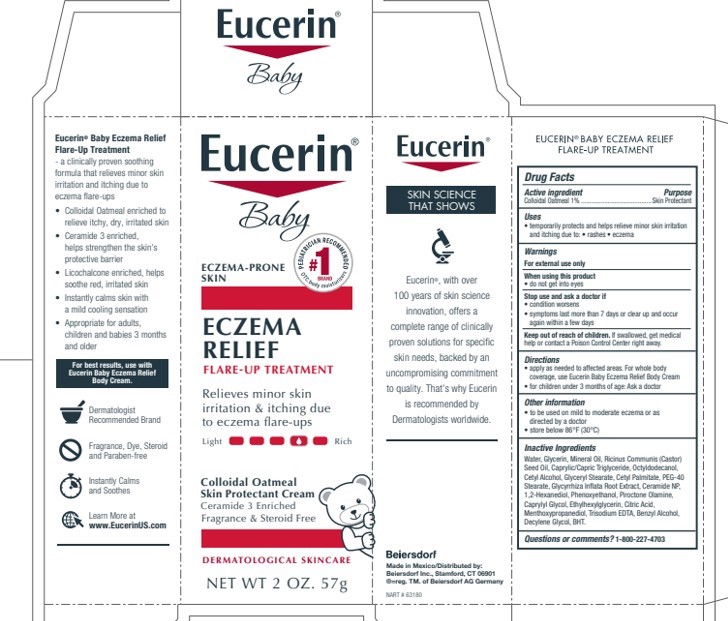
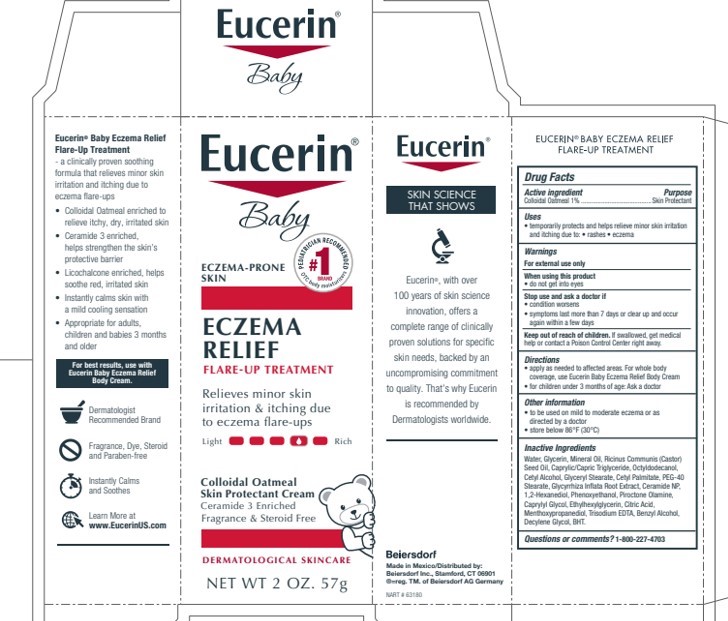
Eucerin Baby
Eczema Relief Flare-Up Treatment
Eczema-Prone Skin
Colloidal Oatmeal Skin Protectant Cream
protects and helps relieve
minor skin irritation and
itching due to eczema flare-ups
Skin Protectant
Steroid Free and Fragrance Free
Dermatological skincare
Number 1 Pediatrician Recommended Brand OTC body moisturizers
-
INGREDIENTS AND APPEARANCE
EUCERIN ECZEMA RELIEF FLARE UP TREATMENT
oatmeal creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10356-371 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OATMEAL (UNII: 8PI54V663Y) (OATMEAL - UNII:8PI54V663Y) OATMEAL 1 g in 100 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) OCTYLDODECANOL (UNII: 461N1O614Y) CETYL PALMITATE (UNII: 5ZA2S6B08X) PEG-40 STEARATE (UNII: ECU18C66Q7) GLYCYRRHIZA INFLATA ROOT (UNII: 1MV1Z7MKVQ) CERAMIDE 3 (UNII: 4370DF050B) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) PHENOXYETHANOL (UNII: HIE492ZZ3T) PIROCTONE OLAMINE (UNII: A4V5C6R9FB) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) BENZYL ALCOHOL (UNII: LKG8494WBH) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 3-((L-MENTHYL)OXY)PROPANE-1,2-DIOL (UNII: KD6TZ2QICH) EDETATE TRISODIUM (UNII: 420IP921MB) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CASTOR OIL (UNII: D5340Y2I9G) MINERAL OIL (UNII: T5L8T28FGP) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) DECYLENE GLYCOL (UNII: S57M60MI88) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10356-371-07 57 g in 1 TUBE; Type 0: Not a Combination Product 06/08/2015 2 NDC:10356-371-25 5 g in 1 TUBE; Type 0: Not a Combination Product 06/08/2015 3 NDC:10356-371-35 141 g in 1 TUBE; Type 0: Not a Combination Product 01/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 06/08/2015 Labeler - Beiersdorf Inc (001177906)