Label: AMORAY INSTANT HAND SANITIZER- ethyl alcohol gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 75749-020-01, 75749-020-02, 75749-020-03, 75749-020-04, view more75749-020-05, 75749-020-06, 75749-020-07, 75749-020-08, 75749-020-09, 75749-020-10, 75749-020-11, 75749-020-12, 75749-020-13, 75749-020-14, 75749-020-15, 75749-020-16, 75749-020-17, 75749-020-18, 75749-020-19, 75749-020-20, 75749-020-21, 75749-020-22, 75749-020-23, 75749-020-24, 75749-020-25, 75749-020-26, 75749-020-27, 75749-020-28, 75749-020-29, 75749-020-30, 75749-020-31, 75749-020-32, 75749-020-33, 75749-020-34, 75749-020-35, 75749-020-36, 75749-020-37, 75749-020-38, 75749-020-39, 75749-020-40, 75749-020-41, 75749-020-42, 75749-020-43, 75749-020-44, 75749-020-45 - Packager: NINGBO PRETTY TOURISM MANUFACTURE CO.,LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 9, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
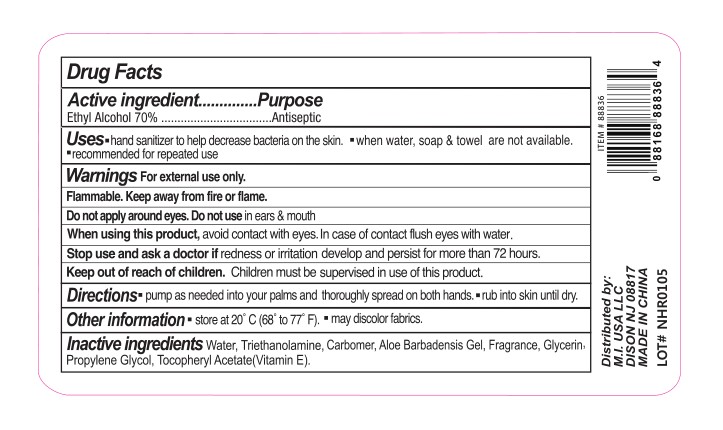
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only. Flammable. Keep away from fire or flame.
When using this product
When using this product, avoid contact with eyes. In case of contact flush eyes with water.
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AMORAY INSTANT HAND SANITIZER
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75749-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) ALOE (UNII: V5VD430YW9) WATER (UNII: 059QF0KO0R) TROLAMINE (UNII: 9O3K93S3TK) 1,2-PROPANEDIOL, 1-BENZOATE (UNII: K4K90ZQ89N) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75749-020-01 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/07/2020 2 NDC:75749-020-02 50 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/07/2020 3 NDC:75749-020-03 54 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/07/2020 4 NDC:75749-020-04 60 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/07/2020 5 NDC:75749-020-05 80 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/07/2020 6 NDC:75749-020-06 100 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/07/2020 7 NDC:75749-020-07 150 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/07/2020 8 NDC:75749-020-08 250 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/07/2020 9 NDC:75749-020-09 300 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/07/2020 10 NDC:75749-020-10 500 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/07/2020 11 NDC:75749-020-11 5 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 07/07/2020 12 NDC:75749-020-12 10 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 07/07/2020 13 NDC:75749-020-13 15 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 07/07/2020 14 NDC:75749-020-14 20 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 07/07/2020 15 NDC:75749-020-15 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 07/07/2020 16 NDC:75749-020-16 50 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 07/07/2020 17 NDC:75749-020-17 60 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 07/07/2020 18 NDC:75749-020-18 100 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 07/07/2020 19 NDC:75749-020-19 150 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 07/07/2020 20 NDC:75749-020-20 250 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 07/07/2020 21 NDC:75749-020-21 300 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 07/07/2020 22 NDC:75749-020-22 500 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 07/07/2020 23 NDC:75749-020-23 237 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/07/2020 24 NDC:75749-020-24 250 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/07/2020 25 NDC:75749-020-25 300 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/07/2020 26 NDC:75749-020-26 350 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/07/2020 27 NDC:75749-020-27 473 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/07/2020 28 NDC:75749-020-28 500 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/07/2020 29 NDC:75749-020-29 750 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/07/2020 30 NDC:75749-020-30 1000 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/07/2020 31 NDC:75749-020-31 2000 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/07/2020 32 NDC:75749-020-32 2500 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/07/2020 33 NDC:75749-020-33 3000 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/07/2020 34 NDC:75749-020-34 4000 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/07/2020 35 NDC:75749-020-35 5000 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/07/2020 36 NDC:75749-020-36 10000 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/07/2020 37 NDC:75749-020-37 30 mL in 1 TUBE; Type 0: Not a Combination Product 07/07/2020 38 NDC:75749-020-38 60 mL in 1 TUBE; Type 0: Not a Combination Product 07/07/2020 39 NDC:75749-020-39 80 mL in 1 TUBE; Type 0: Not a Combination Product 07/07/2020 40 NDC:75749-020-40 100 mL in 1 TUBE; Type 0: Not a Combination Product 07/07/2020 41 NDC:75749-020-41 120 mL in 1 TUBE; Type 0: Not a Combination Product 07/07/2020 42 NDC:75749-020-42 150 mL in 1 TUBE; Type 0: Not a Combination Product 07/07/2020 43 NDC:75749-020-43 180 mL in 1 TUBE; Type 0: Not a Combination Product 07/07/2020 44 NDC:75749-020-44 200 mL in 1 TUBE; Type 0: Not a Combination Product 07/07/2020 45 NDC:75749-020-45 250 mL in 1 TUBE; Type 0: Not a Combination Product 07/07/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 07/07/2020 Labeler - NINGBO PRETTY TOURISM MANUFACTURE CO.,LTD. (553193489) Establishment Name Address ID/FEI Business Operations NINGBO PRETTY TOURISM MANUFACTURE CO.,LTD. 553193489 manufacture(75749-020)