Label: ULTOMIRIS- ravulizumab solution, concentrate
- NDC Code(s): 25682-022-01, 25682-025-01, 25682-028-01
- Packager: Alexion Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated July 5, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ULTOMIRIS safely and effectively. See full prescribing information for ULTOMIRIS.
ULTOMIRIS® (ravulizumab-cwvz) injection, for intravenous use
Initial U.S. Approval: 2018WARNING: SERIOUS MENINGOCOCCAL INFECTIONS
See full prescribing information for complete boxed warning.
ULTOMIRIS increases the risk of serious and life-threatening infections caused by Neisseria meningitidis.
- Complete or update meningococcal vaccination at least 2 weeks prior to the first dose of ULTOMIRIS, unless the risks of delaying ULTOMIRIS outweigh the risks of developing a serious infection. Comply with the most current Advisory Committee on Immunization Practices (ACIP) recommendations for meningococcal vaccination in patients receiving a complement inhibitor. (5.1)
- Patients receiving ULTOMIRIS are at increased risk for invasive disease caused by N. meningitidis, even if they develop antibodies following vaccination. Monitor patients for early signs and symptoms of meningococcal infections and evaluate immediately if infection is suspected. (5.1)
ULTOMIRIS is available only through a restricted program called ULTOMIRIS and SOLIRIS REMS. (5.2)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
ULTOMIRIS is a complement inhibitor indicated for:
- the treatment of adult and pediatric patients one month of age and older with paroxysmal nocturnal hemoglobinuria (PNH). (1.1)
- the treatment of adult and pediatric patients one month of age and older with atypical hemolytic uremic syndrome (aHUS) to inhibit complement-mediated thrombotic microangiopathy (TMA). (1.2)
Limitations of Use:
ULTOMIRIS is not indicated for the treatment of patients with Shiga toxin E. coli related hemolytic uremic syndrome (STEC-HUS). - the treatment of adult patients with generalized myasthenia gravis (gMG) who are anti-acetylcholine receptor (AChR) antibody-positive. (1.3)
- the treatment of adult patients with neuromyelitis optica spectrum disorder (NMOSD) who are anti-aquaporin-4 (AQP4) antibody-positive. (1.4)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
ULTOMIRIS is contraindicated for initiation in patients with unresolved serious Neisseria meningitidis infection. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions in patients with PNH (incidence ≥ 10%) were upper respiratory tract infection and headache. (6.1)
Most common adverse reactions in patients with aHUS (incidence ≥ 20%) were upper respiratory tract infection, diarrhea, nausea, vomiting, headache, hypertension, and pyrexia. (6.1)
Most common adverse reactions in adult patients with gMG (incidence ≥ 10%) were diarrhea and upper respiratory tract infection. (6.1)
Most common adverse reactions in adult patients with NMOSD (incidence ≥ 10%) were COVID-19, headache, back pain, arthralgia, and urinary tract infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Alexion Pharmaceuticals, Inc. at 1-844-259-6783 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 6/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SERIOUS MENINGOCOCCAL INFECTIONS
1 INDICATIONS AND USAGE
1.1 Paroxysmal Nocturnal Hemoglobinuria
1.2 Atypical Hemolytic Uremic Syndrome
1.3 Generalized Myasthenia Gravis
1.4 Neuromyelitis Optica Spectrum Disorder
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage Information
2.2 Recommended Vaccination and Prophylaxis for Meningococcal Infection
2.3 Recommended Dosage for Intravenous Administration in Adult and Pediatric Patients with PNH or aHUS, and in Adult Patients with gMG or NMOSD
2.4 Dosing Considerations
2.5 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Meningococcal Infections
5.2 ULTOMIRIS and SOLIRIS REMS
5.3 Other Infections
5.4 Monitoring Disease Manifestations after ULTOMIRIS Discontinuation
5.5 Thromboembolic Event Management
5.6 Infusion-Related Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Plasma Exchange, Plasmapheresis, and Intravenous Immunoglobulins
7.2 Neonatal Fc Receptor Blockers
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Paroxysmal Nocturnal Hemoglobinuria (PNH)
14.2 Atypical Hemolytic Uremic Syndrome (aHUS)
14.3 Generalized Myasthenia Gravis (gMG)
14.4 Neuromyelitis Optica Spectrum Disorder (NMOSD)
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SERIOUS MENINGOCOCCAL INFECTIONS
ULTOMIRIS, a complement inhibitor, increases the risk of serious infections caused by Neisseria meningitidis [see Warnings and Precautions (5.1)]. Life-threatening and fatal meningococcal infections have occurred in patients treated with complement inhibitors. These infections may become rapidly life-threatening or fatal if not recognized and treated early.
- Complete or update vaccination for meningococcal bacteria (for serogroups A, C, W, Y, and B) at least 2 weeks prior to the first dose of ULTOMIRIS, unless the risks of delaying therapy with ULTOMIRIS outweigh the risk of developing a serious infection. Comply with the most current Advisory Committee on Immunization Practices (ACIP) recommendations for vaccinations against meningococcal bacteria in patients receiving a complement inhibitor. See Warnings and Precautions (5.1) for additional guidance on the management of the risk of serious infections caused by meningococcal bacteria.
- Patients receiving ULTOMIRIS are at increased risk for invasive disease caused by Neisseria meningitidis, even if they develop antibodies following vaccination. Monitor patients for early signs and symptoms of serious meningococcal infections and evaluate immediately if infection is suspected.
Because of the risk of serious meningococcal infections, ULTOMIRIS is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called ULTOMIRIS and SOLIRIS REMS [see Warnings and Precautions (5.2)].
-
1 INDICATIONS AND USAGE
1.1 Paroxysmal Nocturnal Hemoglobinuria
ULTOMIRIS is indicated for the treatment of adult and pediatric patients one month of age and older with paroxysmal nocturnal hemoglobinuria (PNH).
1.2 Atypical Hemolytic Uremic Syndrome
ULTOMIRIS is indicated for the treatment of adult and pediatric patients one month of age and older with atypical hemolytic uremic syndrome (aHUS) to inhibit complement-mediated thrombotic microangiopathy (TMA).
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage Information
ULTOMIRIS is intended to be administered only as an intravenous infusion in adult or pediatric patients one month of age and older.
2.2 Recommended Vaccination and Prophylaxis for Meningococcal Infection
Vaccinate patients against meningococcal infection (serogroups A, C, W, Y and B) according to current ACIP recommendations at least 2 weeks prior to initiation of ULTOMIRIS [see Warnings and Precautions (5.1)].
If urgent ULTOMIRIS therapy is indicated in a patient who is not up to date with meningococcal vaccines according to ACIP recommendations, provide the patient with antibacterial drug prophylaxis and administer these vaccines as soon as possible.
Healthcare providers who prescribe ULTOMIRIS must enroll in the ULTOMIRIS and SOLIRIS REMS [see Warnings and Precautions (5.2)].
2.3 Recommended Dosage for Intravenous Administration in Adult and Pediatric Patients with PNH or aHUS, and in Adult Patients with gMG or NMOSD
The recommended intravenous (IV) ULTOMIRIS loading and maintenance dosing in adult and pediatric patients, one month of age or older weighing 5 kg or greater, with PNH or aHUS, or in adult patients with gMG or NMOSD weighing 40 kg or greater, is based on the patient's body weight, as shown in Table 1, with maintenance doses administered every 4 or 8 weeks, starting 2 weeks after loading dose.
The IV dosing schedule is allowed to occasionally vary within 7 days of the scheduled infusion day (except for the first maintenance dose of ULTOMIRIS); but subsequent doses should be administered according to the original schedule.
Following a missed IV ULTOMIRIS dose, the patient should contact their health care provider immediately.
Table 1: IV Administration of ULTOMIRIS Weight-Based Dosing Regimen – PNH, aHUS, gMG, or NMOSD* Indications Body Weight Range
(kg)Loading Dose
(mg)†Maintenance Dose (mg) and Dosing Interval PNH or aHUS 5 to less than 10 600 300 Every 4 weeks 10 to less than 20 600 600 20 to less than 30 900 2,100 Every 8 weeks
30 to less than 40 1,200 2,700 PNH, aHUS, gMG, or NMOSD 40 to less than 60 2,400 3,000 Every 8 weeks 60 to less than 100 2,700 3,300 100 or greater 3,000 3,600 Refer to Table 2 for treatment initiation instructions in patients who are complement inhibitor treatment-naïve or switching treatment from eculizumab.
Table 2: IV Administration of ULTOMIRIS Treatment Initiation Instructions – PNH, aHUS, gMG, or NMOSD Population Weight-based ULTOMIRIS Loading Dose Time of First ULTOMIRIS Weight-based Maintenance Dose Not currently on ULTOMIRIS or eculizumab treatment At treatment start 2 weeks after ULTOMIRIS loading dose Currently treated with eculizumab At time of next scheduled eculizumab dose 2 weeks after ULTOMIRIS loading dose 2.4 Dosing Considerations
Supplemental Dose of ULTOMIRIS
Plasma exchange (PE), plasmapheresis (PP), and intravenous immunoglobulin (IVIg) have been shown to reduce ULTOMIRIS serum levels. A supplemental dose of ULTOMIRIS is required in the setting of PE, PP, or IVIg (Table 3).
Table 3: Supplemental Dose of ULTOMIRIS after PE, PP, or IVIg* Body Weight Range (kg) Most Recent ULTOMIRIS Dose (mg) Supplemental Dose (mg) following each PE or PP Intervention Supplemental Dose (mg) following Completion of an IVIg Cycle Abbreviations: IVIg = intravenous immunoglobulin; PE = plasma exchange; PP = plasmapheresis - *
- See Table 6 and Table 9 for selection of the proper total volume and maximum infusion rate [see Dosage and Administration (2.5)]
40 to less than 60 2,400 1,200 600 3,000 1,500 60 to less than 100 2,700 1,500 600 3,300 1,800 100 or greater 3,000 1,500 600 3,600 1,800 Timing of ULTOMIRIS Supplemental Dose Within 4 hours following each PE or PP intervention Within 4 hours following completion of an IVIg cycle 2.5 Preparation and Administration
Preparation of ULTOMIRIS Vials for Intravenous Administration
Each vial of ULTOMIRIS is intended for single-dose only.
ULTOMIRIS vials are for intravenous administration by a healthcare provider and are intended for intravenous administration only.
Dilute before use.
Do not mix ULTOMIRIS 100 mg/mL (3 mL and 11 mL vials) and 10 mg/mL (30 mL vial) concentrations together.
Use aseptic technique to prepare ULTOMIRIS as follows:
- 1.
- The number of vials to be diluted is determined based on the individual patient's weight and the prescribed dose [see Dosage and Administration (2.3)].
- 2.
- Prior to dilution, visually inspect the solution in the vials; the solution should be free of any particulate matter or precipitation. Do not use if there is evidence of particulate matter or precipitation.
- 3.
- Withdraw the calculated volume of ULTOMIRIS from the appropriate number of vials and dilute in an infusion bag using 0.9% Sodium Chloride Injection, USP to a final concentration of:
- 50 mg/mL for the 3 mL and 11 mL vial sizes or
- 5 mg/mL for the 30 mL vial size.
The product should be mixed gently. Do not shake. Protect from light. Do not freeze.
Refer to the following reference tables for IV preparation and minimum infusion duration:
-
- ULTOMIRIS 100 mg/mL (3 mL and 11 mL vials): see Table 4 (loading doses), Table 5 (maintenance doses), and Table 6 (supplemental doses)
- ULTOMIRIS 10 mg/mL (30 mL vial): see Table 7 (loading doses), Table 8 (maintenance doses), and Table 9 (supplemental doses)
- 4.
- Administer the prepared solution immediately following preparation.
- 5.
- If the diluted ULTOMIRIS infusion solution is not used immediately, storage under refrigeration at 2°C - 8°C (36°F - 46°F) must not exceed 24 hours taking into account the expected infusion time. Once removed from refrigeration, administer the diluted ULTOMIRIS infusion solution within 6 hours if prepared with ULTOMIRIS 30 mL vials or within 4 hours if prepared with ULTOMIRIS 3 mL or 11 mL vials.
Intravenous Administration of ULTOMIRIS (Healthcare Providers)
Only administer as an intravenous infusion through a 0.2 or 0.22 micron filter.
Dilute ULTOMIRIS to a final concentration of:
-
- 50 mg/mL for the 3 mL and 11 mL vial sizes or
- 5 mg/mL for the 30 mL vial size.
Prior to administration, allow the admixture to adjust to room temperature (18°C - 25°C, 64°F - 77°F). Do not heat the admixture in a microwave or with any heat source other than ambient air temperature.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
After administration of ULTOMIRIS, flush the entire line with 0.9% Sodium Chloride Injection, USP.
Table 4: Loading Dose Reference Table for ULTOMIRIS 100 mg/mL (3 mL and 11 mL Vials) Body Weight Range
(kg)*Loading Dose (mg) ULTOMIRIS
Volume (mL)Volume of NaCl Diluent† (mL) Total Volume (mL) Minimum Infusion Time
(hr)Maximum Infusion Rate
(mL/hr)5 to less than 10‡ 600 6 6 12 1.4 9 10 to less than 20‡ 600 6 6 12 0.8 15 20 to less than 30‡ 900 9 9 18 0.6 30 30 to less than 40‡ 1,200 12 12 24 0.5 48 40 to less than 60 2,400 24 24 48 0.8 60 60 to less than 100 2,700 27 27 54 0.6 90 100 or greater 3,000 30 30 60 0.4 150 Table 5: Maintenance Dose Reference Table for ULTOMIRIS 100 mg/mL (3 mL and 11 mL Vials) Body Weight Range
(kg)*Maintenance Dose (mg) ULTOMIRIS
Volume (mL)Volume of NaCl Diluent† (mL) Total Volume (mL) Minimum Infusion Time
(hr)Maximum Infusion Rate
(mL/hr)5 to less than 10‡ 300 3 3 6 0.8 8 10 to less than 20‡ 600 6 6 12 0.8 15 20 to less than 30‡ 2,100 21 21 42 1.3 33 30 to less than 40‡ 2,700 27 27 54 1.1 50 40 to less than 60 3,000 30 30 60 0.9 67 60 to less than 100 3,300 33 33 66 0.7 95 100 or greater 3,600 36 36 72 0.5 144 Table 6: Supplemental Dose Reference Table for ULTOMIRIS 100 mg/mL (3 mL and 11 mL vials) Body Weight Range (kg)* Supplemental Dose (mg) ULTOMIRIS
Volume (mL)Volume of NaCl Diluent† (mL) Total Volume (mL) Minimum Infusion Time
(hr)Maximum Infusion Rate
(mL/hr)Note: Refer to Table 3 for selection of ravulizumab supplemental dose 40 to less than 60 600 6 6 12 0.25 48 1,200 12 12 24 0.42 57 1,500 15 15 30 0.5 60 60 to less than 100 600 6 6 12 0.20 60 1,500 15 15 30 0.36 83 1,800 18 18 36 0.42 86 100 or greater 600 6 6 12 0.17 71 1,500 15 15 30 0.25 120 1,800 18 18 36 0.28 129 Table 7: Loading Dose Reference Table for ULTOMIRIS 10 mg/mL (30 mL Vial) Body Weight Range
(kg)*Loading Dose (mg) ULTOMIRIS
Volume (mL)Volume of NaCl Diluent† (mL) Total Volume (mL) Minimum Infusion Time
(hr)Maximum Infusion Rate
(mL/hr)5 to less than 10‡ 600 60 60 120 3.8 32 10 to less than 20‡ 600 60 60 120 1.9 64 20 to less than 30‡ 900 90 90 180 1.5 120 30 to less than 40‡ 1,200 120 120 240 1.3 185 40 to less than 60 2,400 240 240 480 1.9 253 60 to less than 100 2,700 270 270 540 1.7 318 100 or greater 3,000 300 300 600 1.8 334 Table 8: Maintenance Dose Reference Table for ULTOMIRIS 10 mg/mL (30 mL Vial) Body Weight Range
(kg)*Maintenance Dose (mg) ULTOMIRIS
Volume (mL)Volume of NaCl Diluent† (mL) Total Volume (mL) Minimum Infusion Time
(hr)Maximum Infusion Rate
(mL/hr)5 to less than 10‡ 300 30 30 60 1.9 32 10 to less than 20‡ 600 60 60 120 1.9 64 20 to less than 30‡ 2,100 210 210 420 3.3 128 30 to less than 40‡ 2,700 270 270 540 2.8 193 40 to less than 60 3,000 300 300 600 2.3 261 60 to less than 100 3,300 330 330 660 2 330 100 or greater 3,600 360 360 720 2.2 328 Table 9: Supplemental Dose Reference Table for ULTOMIRIS 10 mg/mL (30 mL vial) Body Weight Range (kg)* Supplemental Dose (mg) ULTOMIRIS
Volume (mL)Volume of NaCl Diluent† (mL) Total Volume (mL) Minimum Infusion Time
(hr)Maximum Infusion Rate
(mL/hr)Note: Refer to Table 3 for selection of ravulizumab supplemental dose 40 to less than 60 600 60 60 120 0.5 240 1,200 120 120 240 1 240 1,500 150 150 300 1.2 250 60 to less than 100 600 60 60 120 0.4 300 1,500 150 150 300 1 300 1,800 180 180 360 1.1 327 100 or greater 600 60 60 120 0.4 300 1,500 150 150 300 1 300 1,800 180 180 360 1.1 327 If an adverse reaction occurs during the administration of ULTOMIRIS, the infusion may be slowed or stopped at the discretion of the physician. Monitor the patient for at least 1 hour following completion of the infusion for signs or symptoms of an infusion-related reaction.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
ULTOMIRIS is contraindicated for initiation in patients with unresolved serious Neisseria meningitidis infection [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Meningococcal Infections
ULTOMIRIS, a complement inhibitor, increases a patient's susceptibility to serious, life-threatening, or fatal infections caused by meningococcal bacteria (septicemia and/or meningitis) in any serogroup, including non-groupable strains. Life-threatening and fatal meningococcal infections have occurred in both vaccinated and unvaccinated patients treated with complement inhibitors. The initiation of ULTOMIRIS treatment is contraindicated in patients with unresolved serious Neisseria meningitidis infection.
Complete or update meningococcal vaccination (for serogroups A, C, W, Y and B) at least 2 weeks prior to administration of the first dose of ULTOMIRIS, according to current ACIP recommendations for patients receiving a complement inhibitor. Revaccinate patients in accordance with ACIP recommendations considering the duration of ULTOMIRIS therapy. Note that ACIP recommends an administration schedule in patients receiving complement inhibitors that differs from the administration schedule in the vaccine prescribing information. If urgent ULTOMIRIS therapy is indicated in a patient who is not up to date with meningococcal vaccines according to ACIP recommendations, provide the patient with antibacterial drug prophylaxis and administer meningococcal vaccines as soon as possible. Various durations and regimens of antibacterial drug prophylaxis have been considered, but the optimal durations and drug regimens for prophylaxis and their efficacy have not been studied in unvaccinated or vaccinated patients receiving complement inhibitors, including ULTOMIRIS. The benefits and risks of treatment with ULTOMIRIS, as well as the benefits and risks of antibacterial drug prophylaxis in unvaccinated or vaccinated patients, must be considered against the known risks for serious infections caused by Neisseria meningitidis.
Vaccination does not eliminate the risk of meningococcal infections, despite development of antibodies following vaccination.
Closely monitor patients for early signs and symptoms of meningococcal infection and evaluate patients immediately if infection is suspected. Inform patients of these signs and symptoms and instruct patients to seek immediate medical care if these signs and symptoms occur. Promptly treat known infections. Meningococcal infection may become rapidly life-threatening or fatal if not recognized and treated early. Consider interruption of ULTOMIRIS in patients who are undergoing treatment for serious meningococcal infection, depending on the risks of interrupting treatment in the disease being treated.
ULTOMIRIS is available only through a restricted program under a REMS [see Warnings and Precautions (5.2)].
5.2 ULTOMIRIS and SOLIRIS REMS
ULTOMIRIS is available only through a restricted program under a REMS called ULTOMIRIS and SOLIRIS REMS, because of the risk of serious meningococcal infections [see Warnings and Precautions (5.1)].
Notable requirements of the ULTOMIRIS and SOLIRIS REMS include the following:
- Prescribers must enroll in the REMS.
- Prescribers must counsel patients about the risk of serious meningococcal infection.
- Prescribers must provide the patients with the REMS educational materials.
- Prescribers must assess patient vaccination status for meningococcal vaccines (against serogroups A, C, W, Y, and B) and vaccinate if needed according to current ACIP recommendations two weeks prior to the first dose of ULTOMIRIS.
- Prescribers must provide a prescription for antibacterial drug prophylaxis if treatment must be started urgently and the patient is not up to date with meningococcal vaccines according to current ACIP recommendations at least two weeks prior to the first dose of ULTOMIRIS.
- Healthcare settings and pharmacies that dispense ULTOMIRIS must be certified in the REMS and must verify prescribers are certified.
- Patients must receive counseling from the prescriber about the need to receive meningococcal vaccines per ACIP recommendations, the need to take antibiotics as directed by the prescriber, and the signs and symptoms of meningococcal infection.
- Patients must be instructed to carry the Patient Safety Card with them at all times during and for 8 months following treatment with ULTOMIRIS.
Further information is available at www.UltSolREMS.com or 1-888-765-4747.
5.3 Other Infections
Serious infections with Neisseria species (other than Neisseria meningitidis), including disseminated gonococcal infections, have been reported.
ULTOMIRIS blocks terminal complement activation; therefore, patients may have increased susceptibility to infections, especially with encapsulated bacteria, such as infections caused by Neisseria meningitidis but also Streptococcus pneumoniae, Haemophilus influenzae, and to a lesser extent, Neisseria gonorrhoeae. Children treated with ULTOMIRIS may be at increased risk of developing serious infections due to Streptococcus pneumoniae and Haemophilus influenzae type b (Hib). Administer vaccinations for the prevention of Streptococcus pneumoniae and Haemophilus influenzae type b (Hib) infections according to ACIP recommendations. Patients receiving ULTOMIRIS are at increased risk for infections due to these organisms, even if they develop antibodies following vaccination.
5.4 Monitoring Disease Manifestations after ULTOMIRIS Discontinuation
Treatment Discontinuation for PNH
After discontinuing treatment with ULTOMIRIS, closely monitor for signs and symptoms of hemolysis, identified by elevated lactate dehydrogenase (LDH) along with sudden decrease in PNH clone size or hemoglobin, or reappearance of symptoms such as fatigue, hemoglobinuria, abdominal pain, shortness of breath (dyspnea), major adverse vascular event (including thrombosis), dysphagia, or erectile dysfunction. Monitor any patient who discontinues ULTOMIRIS for at least 16 weeks to detect hemolysis and other reactions. If signs and symptoms of hemolysis occur after discontinuation, including elevated LDH, consider restarting treatment with ULTOMIRIS.
Treatment Discontinuation for aHUS
ULTOMIRIS treatment of aHUS should be a minimum duration of 6 months. Due to heterogeneous nature of aHUS events and patient-specific risk factors, treatment duration beyond the initial 6 months should be individualized.
There are no specific data on ULTOMIRIS discontinuation.
After discontinuing treatment with ULTOMIRIS, patients should be monitored for clinical symptoms and laboratory signs of TMA complications for at least 12 months.
TMA complications post-discontinuation can be identified if any of the following is observed:
- Clinical symptoms of TMA include changes in mental status, seizures, angina, dyspnea, thrombosis or increasing blood pressure.
- In addition, at least two of the following laboratory signs observed concurrently and results should be confirmed by a second measurement 28 days apart with no interruption:
- a decrease in platelet count of 25% or more as compared to either baseline or to peak platelet count during ULTOMIRIS treatment;
- an increase in serum creatinine of 25% or more as compared to baseline or to nadir during ULTOMIRIS treatment;
- an increase in serum LDH of 25% or more as compared to baseline or to nadir during ULTOMIRIS treatment.
If TMA complications occur after ULTOMIRIS discontinuation, consider reinitiation of ULTOMIRIS treatment or appropriate organ-specific supportive measures.
5.5 Thromboembolic Event Management
The effect of withdrawal of anticoagulant therapy during ULTOMIRIS treatment has not been established. Therefore, treatment with ULTOMIRIS should not alter anticoagulant management.
5.6 Infusion-Related Reactions
Administration of ULTOMIRIS may result in systemic infusion-related reactions, including anaphylaxis [see Adverse Reactions (6.2)] and hypersensitivity reactions. In clinical trials, infusion-related reactions occurred in approximately 1 to 7% of patients treated with ULTOMIRIS [see Adverse Reactions (6.1)]. These events included lower back pain, abdominal pain, muscle spasms, drop in blood pressure, elevation in blood pressure, rigors, limb discomfort, drug hypersensitivity (allergic reaction), and dysgeusia (bad taste). These reactions did not require discontinuation of ULTOMIRIS. If signs of cardiovascular instability or respiratory compromise occur, interrupt ULTOMIRIS infusion and institute appropriate supportive measures.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Serious Meningococcal Infections [see Warnings and Precautions (5.1)]
- Other Infections [see Warnings and Precautions (5.3)]
- Infusion-Related Reactions [see Warnings and Precautions (5.6)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Paroxysmal Nocturnal Hemoglobinuria (PNH)
Adult Population with PNH Treated with ULTOMIRIS
The data described below reflect exposure of 441 adult patients with PNH in Phase 3 studies who received ULTOMIRIS (n = 222) or eculizumab (n = 219) at the recommended dosing regimens with median treatment duration of 6 months for ULTOMIRIS and 6 months for eculizumab. The most frequent adverse reactions (≥ 10%) with ULTOMIRIS were upper respiratory tract infection and headache. Table 10 describes adverse reactions that occurred at a rate of 5% or more among patients treated with ULTOMIRIS in PNH studies.
Serious adverse reactions were reported in 15 (6.8%) patients with PNH receiving ULTOMIRIS. The serious adverse reactions in patients treated with ULTOMIRIS included hyperthermia and pyrexia. No serious adverse reaction was reported in more than 1 patient treated with ULTOMIRIS.
One fatal case of sepsis was identified in a patient treated with ULTOMIRIS.
Table 10: Adverse Reactions Reported in 5% or More of ULTOMIRIS-Treated Patients in Complement Inhibitor Naïve and Eculizumab-Experienced Adult Patients with PNH Body System
Adverse ReactionNumber of Patients ULTOMIRIS
(N=222)
n (%)Eculizumab
(N=219)
n (%)- *
- Grouped term includes: nasopharyngitis, upper respiratory tract infection, oropharyngeal pain, viral upper respiratory tract infection, rhinitis, respiratory tract infection, rhinorrhea, pharyngitis, and upper respiratory tract inflammation
Gastrointestinal disorders Diarrhea 19 (9) 12 (5) Nausea 19 (9) 19 (9) Abdominal pain 13 (6) 16 (7) General disorders and administration site conditions Pyrexia 15 (7) 18 (8) Infections and infestations Upper respiratory tract infection* 86 (39) 86 (39) Musculoskeletal and connective tissue disorders Pain in extremity 14 (6) 11 (5) Arthralgia 11 (5) 12 (5) Nervous system disorders Headache 71 (32) 57 (26) Dizziness 12 (5) 14 (6) Clinically relevant adverse reactions in 1% of patients include infusion-related reactions.
Pediatric Population with PNH Treated with ULTOMIRIS
In pediatric patients with PNH (aged 9 to 17 years old) included in the pediatric PNH Phase 3 study, the safety profile appeared similar to that observed in adult patients with PNH and in pediatric and adult patients with aHUS. The most common adverse reactions (> 20%) were upper respiratory tract infection, anemia, abdominal pain, and headache. Table 11 describes the adverse reactions that occurred at a rate of 10% or more among pediatric patients treated with ULTOMIRIS in Study ALXN1210-PNH-304.
Table 11: Adverse Reactions Reported in 10% or More of ULTOMIRIS-Treated Pediatric Patients with PNH in Study ALXN1210-PNH-304 Body System
Adverse ReactionTreatment Naïve
(N=5)Eculizumab Experienced
(N=8)Total
(N=13)n (%) n (%) n (%) Blood and lymphatic system disorders Anemia* 1 (20) 2 (25) 3 (23) Gastrointestinal disorders Abdominal pain 0 (0) 3 (38) 3 (23) Constipation 0 (0) 2 (25) 2 (15) General disorders and administration site conditions Pyrexia 1 (20) 1 (13) 2 (15) Infections and infestations Upper Respiratory tract infection† 1 (20) 6 (75) 7 (54) Musculoskeletal and connective tissue disorders Pain in extremity 0 (0) 2 (25) 2 (15) Nervous system disorders Headache 1 (20) 2 (25) 3 (23) Atypical Hemolytic Uremic Syndrome (aHUS)
The data described below reflect exposure of 58 adult and 16 pediatric patients with aHUS in single-arm trials who received ULTOMIRIS at the recommended dose and schedule. The most frequent adverse reactions reported in ≥ 20% of patients treated with ULTOMIRIS were upper respiratory tract infection, diarrhea, nausea, vomiting, headache, hypertension, and pyrexia. Table 12, Table 13 and Table 14 describe adverse reactions that occurred at a rate of 10% or more among patients treated with ULTOMIRIS in aHUS studies. Serious adverse reactions were reported in 42 (57%) patients with aHUS receiving ULTOMIRIS. The most frequent serious adverse reactions reported in more than 2 patients (2.7%) treated with ULTOMIRIS were hypertension, pneumonia, and abdominal pain. Four patients died during the ALXN1210-aHUS-311 study. The cause of death was sepsis in 2 patients and intracranial hemorrhage in 1 patient. The fourth patient, who was excluded from the trial after a diagnosis of STEC-HUS, died due to pretreatment cerebral arterial thrombosis.
Table 12: Adverse Reactions Reported in ≥ 10% of ULTOMIRIS-Treated Patients with aHUS in Study ALXN1210-aHUS-311 Body System
Adverse ReactionALXN1210-aHUS-311
(N=58)All Grades*
(n=53)
n (%)≥ Grade 3
(n=14)
n (%)- *
- Graded per CTCAE v5.0.
- †
- Grouped term includes nasopharyngitis, pharyngitis, upper respiratory tract infection, rhinitis, viral upper respiratory tract infection, rhinovirus infection, viral pharyngitis, rhinorrhea, and oropharyngeal pain.
- ‡
- Grouped term includes gastroenteritis, gastrointestinal infection, enterocolitis infectious, infectious colitis, and enterocolitis.
Blood and lymphatic system disorders Anemia 8 (14) 0 (0) Gastrointestinal disorders Diarrhea 18 (31) 2 (3) Nausea 15 (26) 2 (3) Vomiting 15 (26) 2 (3) Constipation 8 (14) 1 (2) Abdominal pain 7 (12) 1 (2) General disorders and administration site conditions Pyrexia 11 (19) 1 (2) Edema peripheral 10 (17) 0 (0) Fatigue 8 (14) 0 (0) Infections and infestations Upper respiratory tract infection† 15 (26) 0 (0) Urinary tract infection 10 (17) 5 (9) Gastrointestinal infection‡ 8 (14) 2 (3) Metabolism and nutrition disorders Hypokalemia 6 (10) 1 (2) Musculoskeletal and connective tissue disorders Arthralgia 13 (22) 0 (0) Back pain 7 (12) 1 (2) Muscle spasms 6 (10) 0 (0) Pain in extremity 6 (10) 0 (0) Nervous system disorders Headache 23 (40) 1 (2) Psychiatric disorders Anxiety 8 (14) 1 (2) Respiratory, thoracic and mediastinal disorders Cough 10 (17) 0 (0) Dyspnea 10 (17) 1 (2) Skin and subcutaneous tissue disorders Alopecia 6 (10) 0 (0) Dry skin 6 (10) 0 (0) Vascular disorders Hypertension 14 (24) 7 (12) Clinically relevant adverse reactions include viral tonsilitis (in < 10% of patients) and infusion-related reactions (in 3% of patients).
Table 13: Adverse Reactions Reported in ≥ 10% of ULTOMIRIS-Treated Patients with aHUS in Study ALXN1210-aHUS-312 Body System
Adverse ReactionALXN1210-aHUS-312
(N=16)All Grades*
(n=16)
n (%)≥ Grade 3
(n=6)
n (%)Blood and lymphatic system disorders Anemia 2 (13) 1 (6) Lymphadenopathy 2 (13) 0 (0) Gastrointestinal disorders Diarrhea 6 (38) 0 (0) Constipation 4 (25) 0 (0) Vomiting 4 (25) 1 (6) Abdominal pain 3 (19) 0 (0) Nausea 2 (13) 0 (0) General disorders and administration site conditions Pyrexia 8 (50) 0 (0) Infections and infestations Upper respiratory tract infection† 7 (44) 1 (6) Gastroenteritis viral 2 (13) 2 (13) Pneumonia 2 (13) 1 (6) Tonsillitis 2 (13) 0 (0) Injury, poisoning and procedural complications Contusion 3 (19) 0 (0) Investigations Vitamin D decreased 3 (19) 0 (0) Metabolism and nutrition disorders Decreased appetite 2 (13) 0 (0) Iron deficiency 2 (13) 0 (0) Musculoskeletal and connective tissue disorders Myalgia 3 (19) 0 (0) Pain in extremity 2 (13) 0 (0) Nervous system disorders Headache 5 (31) 0 (0) Respiratory, thoracic and mediastinal disorders Cough 3 (19) 0 (0) Dyspnea 2 (13) 0 (0) Skin and subcutaneous tissue disorders Rash 3 (19) 0 (0) Vascular disorders Hypertension 4 (25) 1 (6) Hypotension 2 (13) 0 (0) Clinically relevant adverse reactions in < 10% of patients include viral infection.
Table 14: Adverse Reactions Reported in ≥ 10% of ULTOMIRIS-Treated Patients from Birth to 16 Years of Age with aHUS in Study ALXN1210-aHUS-312 Body System
Adverse ReactionALXN1210-aHUS-312 Age 0 to < 2
(N=2)Age 2 to < 12
(N=12)Age 12 to 16
(N=1)Total
(N=15)n (%) n (%) n (%) n (%) - *
- Grouped term includes nasopharyngitis, pharyngitis, upper respiratory tract infection, rhinitis, viral upper respiratory tract infection, rhinovirus infection, viral pharyngitis, rhinorrhea, and oropharyngeal pain
Blood and lymphatic system disorders Lymphadenopathy 0 (0) 2 (17) 0 (0) 2 (13) Gastrointestinal disorders Diarrhea 1 (50) 3 (25) 1 (100) 5 (33) Constipation 0 (0) 4 (33) 0 (0) 4 (27) Vomiting 0 (0) 3 (25) 0 (0) 3 (20) Abdominal pain 0 (0) 2 (17) 0 (0) 2 (13) General disorders and administration site conditions Pyrexia 1 (50) 5 (42) 1 (100) 7 (47) Infections and infestations Upper respiratory tract infection* 1 (50) 6 (50) 0 (0) 7 (47) Gastroenteritis viral 0 (0) 2 (17) 0 (0) 2 (13) Tonsillitis 1 (50) 1 (8) 0 (0) 2 (13) Injury, poisoning and procedural complications Contusion 0 (0) 2 (17) 0 (0) 2 (13) Investigations Vitamin D decreased 0 (0) 2 (17) 1 (100) 3 (20) Metabolism and nutrition disorders Decreased appetite 1 (50) 1 (8) 0 (0) 2 (13) Iron deficiency 0 (0) 2 (17) 0 (0) 2 (13) Musculoskeletal and connective tissue disorders Myalgia 1 (50) 1 (8) 0 (0) 2 (13) Pain in extremity 0 (0) 2 (17) 0 (0) 2 (13) Nervous system disorders Headache 0 (0) 4 (33) 0 (0) 4 (27) Respiratory, thoracic and mediastinal disorders Cough 0 (0) 3 (25) 0 (0) 3 (20) Dyspnea 1 (50) 1 (8) 0 (0) 2 (13) Skin and subcutaneous tissue disorders Rash 1 (50) 2 (17) 0 (0) 3 (20) Vascular disorders Hypertension 1 (50) 3 (25) 0 (0) 4 (27) Hypotension 0 (0) 2 (17) 0 (0) 2 (13) Clinically relevant adverse reactions in < 10% of patients include viral infection.
Generalized Myasthenia Gravis (gMG)
The safety of ULTOMIRIS has been evaluated in 175 adult patients with gMG, including 169 patients who received at least one dose of ULTOMIRIS, 142 patients who were exposed for at least 6 months, and 95 who were exposed for at least 12 months [see Clinical Studies (14.3)]. In a randomized, double-blind, placebo-controlled trial (ALXN1210-MG-306), the most frequent adverse reactions (≥ 10%) with ULTOMIRIS were diarrhea and upper respiratory tract infection. Table 15 describes adverse reactions that occurred at a rate of 5% or more and at greater frequency than placebo. Serious adverse reactions were reported in 20 (23%) patients with gMG receiving ULTOMIRIS and in 14 (16%) patients receiving placebo. The most frequent serious adverse reactions were infections reported in at least 8 (9%) patients treated with ULTOMIRIS and in 4 (4%) patients treated with placebo [see Warnings and Precautions (5.3)]. Of these infections, one fatal case of COVID-19 pneumonia was identified in a patient treated with ULTOMIRIS and one case of infection led to discontinuation of ULTOMIRIS.
Table 15: Adverse Reactions Reported in ≥ 5% and at Greater Frequency than Placebo in ULTOMIRIS-Treated Adult Patients with gMG in Study ALXN1210-MG-306 Body System
Adverse ReactionNumber of Patients ULTOMIRIS
(N=86)
n (%)Placebo
(N=89)
n (%)Gastrointestinal Disorders Diarrhea 13 (15) 11 (12) Abdominal pain 5 (6) 0 Infections and Infestations Upper respiratory tract infection 12 (14) 7 (8) Urinary tract infection 5 (6) 4 (4) Musculoskeletal and Connective Tissue Disorders Back Pain 7 (8) 5 (6) Nervous System Disorders Dizziness 8 (9) 3 (3) Neuromyelitis Optica Spectrum Disorder (NMOSD)
The safety of ULTOMIRIS has been evaluated in 58 adult patients with NMOSD who received at least one dose of ULTOMIRIS [see Clinical Studies (14.3)]. In Study ALXN1210-NMO-307, an open-label multicenter trial, the most frequent adverse reactions (≥10%) with ULTOMIRIS were COVID-19, headache, back pain, urinary tract infection and arthralgia.
Table 16 describes adverse reactions that occurred at a rate of 5% or more in patients treated with ULTOMIRIS. Serious adverse reactions were reported in 8 (13.8%) patients with NMOSD receiving ULTOMIRIS.
Table 16: Adverse Reactions Reported in ≥ 5% in ULTOMIRIS-Treated Adult Patients with NMOSD in Study ALXN1210-NMO-307 Body System
Adverse ReactionULTOMIRIS
(N=58)
n (%)Blood and Lymphatic System Disorder Lymphadenopathy 3 (5) Gastrointestinal Disorders Constipation 4 (7) Vomiting 4 (7) Diarrhea 3 (5) Gastroesophageal reflux disease 3 (5) General Disorders and Administration Site Reactions Pyrexia 5 (9) Chills 3 (5) Fatigue 3 (5) Malaise 3 (5) Non-cardiac chest pain 3 (5) Vaccination site pain 3 (5) Infections and Infestations COVID-19 14 (24) Urinary tract infection 6 (10) Cystitis 5 (9) Upper respiratory tract infection 5 (9) Nasopharyngitis 3 (5) Sinusitis 3 (5) Injury, Poisoning and Procedural Complications Infusion related reaction 4 (7) Musculoskeletal and Connective Tissue Disorders Back pain 7 (12) Arthralgia 6 (10) Myalgia 3 (5) Nervous System Disorders Headache 14 (24) Dizziness 4 (7) Migraine 3 (5) Respiratory, thoracic and mediastinal disorders Cough 3 (5) 6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ULTOMIRIS. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to ULTOMIRIS exposure.
Serious Adverse Reaction: Anaphylaxis [see Warnings and Precautions (5.6)]
-
7 DRUG INTERACTIONS
7.1 Plasma Exchange, Plasmapheresis, and Intravenous Immunoglobulins
Concomitant use of ULTOMIRIS with plasma exchange (PE), plasmapheresis (PP), or intravenous immunoglobulin (IVIg) treatment can reduce serum ravulizumab concentrations and requires a supplemental dose of ULTOMIRIS [see Dosage and Administration (2.5)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ULTOMIRIS during pregnancy. Healthcare providers and patients may call 1-833-793-0563 or go to www.UltomirisPregnancyStudy.com to enroll in or to obtain information about the registry.
Risk Summary
There are no available data on ULTOMIRIS use in pregnant women to inform a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. There are risks to the mother and fetus associated with untreated PNH and aHUS in pregnancy (see Clinical Considerations). Animal studies using a mouse analogue of the ravulizumab-cwvz molecule (murine anti-mouse complement component 5 [C5] antibody) showed increased rates of developmental abnormalities and an increased rate of dead and moribund offspring at doses 0.8-2.2 times the human dose (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated Maternal and/or Fetal/neonatal Risk
PNH in pregnancy is associated with adverse maternal outcomes, including worsening cytopenias, thrombotic events, infections, bleeding, miscarriages, and increased maternal mortality, and adverse fetal outcomes, including fetal death and premature delivery.
In pregnancy, aHUS is associated with adverse maternal outcomes, including preeclampsia and preterm delivery, and adverse fetal/neonatal outcomes, including intrauterine growth restriction (IUGR), fetal death and low birth weight.
Data
Animal Data
Animal reproduction studies were conducted in mice using doses of a murine anti-C5 antibody that approximated 1-2.2 times (loading dose) and 0.8-1.8 times (maintenance dose) the recommended human ULTOMIRIS dose, based on a body weight comparison. When animal exposure to the antibody occurred in the time period from before mating until early gestation, no decrease in fertility or reproductive performance was observed. When maternal exposure to the antibody occurred during organogenesis, 2 cases of retinal dysplasia and 1 case of umbilical hernia were observed among 230 offspring born to mothers exposed to the higher antibody dose; however, the exposure did not increase fetal loss or neonatal death. When maternal exposure to the antibody occurred in the time period from implantation through weaning, a higher number of male offspring became moribund or died (1/25 controls, 2/25 low dose group, 5/25 high dose group). Surviving offspring had normal development and reproductive function. Human IgG are known to cross the human placental barrier, and thus ULTOMIRIS may potentially cause terminal complement inhibition in fetal circulation.
8.2 Lactation
Risk summary
There are no data on the presence of ravulizumab-cwvz in human milk, the effect on the breastfed child, or the effect on milk production. Since many medicinal products and immunoglobulins are secreted into human milk, and because of the potential for serious adverse reactions in a nursing child, breastfeeding should be discontinued during treatment and for 8 months after the final dose.
8.4 Pediatric Use
The safety and effectiveness of ULTOMIRIS for the treatment of PNH have been established in pediatric patients aged one month and older. Use of ULTOMIRIS for this indication is supported by evidence from adequate and well-controlled trials in adults with additional pharmacokinetic, efficacy and safety data in pediatric patients aged 9 to 17 years [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.1)]. The safety and efficacy for the treatment of pediatric and adult patients with PNH appear similar. Use of ULTOMIRIS in pediatric patients with PNH aged less than 9 years and body weight < 30 kg is based on extrapolation of pharmacokinetic / pharmacodynamic (PK/PD), and efficacy and safety data from aHUS and PNH clinical studies [see Clinical Pharmacology (12.3) and Clinical Studies (14)].
The safety and effectiveness of ULTOMIRIS for the treatment of aHUS have been established in pediatric patients aged one month and older. Use of ULTOMIRIS for this indication is supported by evidence from adequate and well-controlled studies in adults with additional pharmacokinetic, safety, and efficacy data in pediatric patients aged 10 months to < 17 years. The safety and efficacy of ULTOMIRIS for the treatment of aHUS appear similar in pediatric and adult patients [see Adverse Reactions (6.1), and Clinical Studies (14.2)].
The safety and effectiveness of ULTOMIRIS for the treatment of gMG or NMOSD in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of ULTOMIRIS did not include sufficient numbers of subjects aged 65 and over (58 patients with PNH, 9 with aHUS, 54 with gMG, and 7 with NMOSD) to determine whether they respond differently from younger subjects.
Other reported clinical experience has not identified differences in responses between elderly and younger patients.
-
11 DESCRIPTION
Ravulizumab-cwvz, a complement inhibitor, is a humanized monoclonal antibody (mAb) produced in Chinese hamster ovary (CHO) cells. Ravulizumab-cwvz consists of 2 identical 448 amino acid heavy chains and 2 identical 214 amino acid light chains and has a molecular weight of approximately 148 kDa. The constant regions of ravulizumab-cwvz include the human kappa light chain constant region, and the protein engineered "IgG2/4" heavy chain constant region.
The heavy chain CH1 domain, hinge region, and the first 5 amino acids of the CH2 domain match the human IgG2 amino acid sequence, residues 6 to 36 in the CH2 region (common to both human IgG2 and IgG4 amino acid sequences), while the remainder of the CH2 domain and the CH3 domain match the human IgG4 amino acid sequence. The heavy and light chain variable regions that form the human C5 binding site consist of human framework regions grafted to murine complementarity-determining regions.
ULTOMIRIS 100 mg/mL (3 mL and 11 mL vials)
ULTOMIRIS (ravulizumab-cwvz) injection 100 mg/mL is a sterile, translucent, clear to yellowish color, preservative-free solution for intravenous use. Each single-dose vial contains 300 mg or 1,100 mg ravulizumab-cwvz at a concentration of 100 mg/mL with a pH of 7.4. Each mL also contains L-arginine (4.33 mg), polysorbate 80 (0.5 mg) (vegetable origin), sodium phosphate dibasic (4.42 mg), sodium phosphate monobasic (4.57 mg), sucrose (50 mg), and Water for Injection, USP.
ULTOMIRIS 10 mg/mL (30 mL vial)
ULTOMIRIS (ravulizumab-cwvz) injection 10 mg/mL is a sterile, clear to translucent, slight whitish color, preservative-free solution for intravenous use. Each single-dose vial contains 300 mg ravulizumab-cwvz at a concentration of 10 mg/mL with a pH of 7.0. Each mL also contains polysorbate 80 (0.2 mg) (vegetable origin), sodium chloride (8.77 mg), sodium phosphate dibasic (1.78 mg), sodium phosphate monobasic (0.46 mg), and Water for Injection, USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ravulizumab-cwvz is a terminal complement inhibitor that specifically binds to the complement protein C5 with high affinity, thereby inhibiting its cleavage to C5a (the proinflammatory anaphylatoxin) and C5b (the initiating subunit of the membrane attack complex [MAC or C5b-9]) thus preventing MAC formation. ULTOMIRIS inhibits terminal complement-mediated intravascular hemolysis in patients with PNH and complement-mediated thrombotic microangiopathy (TMA) in patients with aHUS.
The precise mechanism by which ravulizumab-cwvz exerts its therapeutic effect in gMG patients is unknown, but is presumed to involve reduction of terminal complement complex C5b-9 deposition at the neuromuscular junction.
The precise mechanism by which ravulizumab-cwvz exerts its therapeutic effect in NMOSD is unknown, but is presumed to involve inhibition of aquaporin-4 antibody-induced terminal complement C5b-9 deposition.
12.2 Pharmacodynamics
Complete inhibition of serum free C5 (concentration of less than 0.5 mcg/mL) was observed by the end of the first ULTOMIRIS infusion and sustained throughout the entire 26-week treatment period in both adult and pediatric patients with PNH, in the majority (93%) of adult and pediatric patients with aHUS, in all adult patients with gMG, and in the majority (98.3%) of adult patients with NMOSD.
The extent and duration of the pharmacodynamic response in patients with PNH, aHUS, gMG, or NMOSD were exposure-dependent for ULTOMIRIS. Free C5 levels of < 0.5 mcg/mL were correlated with maximal intravascular hemolysis control and complete terminal complement inhibition in patients with PNH.
Complete terminal complement inhibition following initiation of ULTOMIRIS treatment led to normalization of serum LDH by week 4 in complement-inhibitor naïve patients with PNH, and maintained LDH normalization in patients previously treated with eculizumab with PNH [see Clinical Studies (14)].
12.3 Pharmacokinetics
Following ULTOMIRIS treatment, ravulizumab-cwvz pharmacokinetics increase proportionally over a dose range of 200 to 5400 mg. Ravulizumab-cwvz Cmax and Ctrough parameters are presented in Table 17, Table 18, Table 19, and Table 20.
Table 17: Mean (%CV) Pharmacokinetic Parameters Following ULTOMIRIS Treatment in Patients with PNH who are Complement Inhibitor-Naïve and Patients Previously Treated with Eculizumab Pediatric Patients Adult Patients ALXN1210-PNH-304 ALXN1210-PNH-301 ALXN1210-PNH-302 N Complement Inhibitor-Naïve N Previously Treated with Eculizumab N Complement Inhibitor-Naïve N Previously Treated with Eculizumab Abbreviations: LD = Loading Dose; MD = Maintenance Dose Cmax
(mcg/mL)LD 4 733 (14.5) 8 885 (19.3) 125 771 (21.5) 95 843 (24.1) MD 4 1490 (26.7) 8 1705 (9.7) 124 1,379 (20.0) 95 1,386 (19.4) Ctrough
(mcg/mL)LD 4 368 (14.7) 8 452 (15.1) 125 391 (35.0) 96 405 (29.9) MD 4 495 (21.3) 8 566 (12.2) 124 473 (33.4) 95 501 (28.6) Table 18: Mean (%CV) Pharmacokinetic Parameters Following ULTOMIRIS Treatment in Patients with aHUS Pediatric Patients
(ALXN1210-aHUS-312)Adult Patients
(ALXN1210-aHUS-311)N < 20 kg
MD Q4WN ≥ 20 to < 40 kg
MD Q8WN ≥ 40 kg
MD Q8WAbbreviations: LD = Loading Dose; MD = Maintenance Dose; Q4W = Every 4 Weeks; Q8W = Every 8 Weeks Cmax
(mcg/mL)LD 8 656 (38.1) 4 600 (17.3) 52 754 (35.2) MD 7 1,467 (37.8) 6 1,863 (15.3) 46 1,458 (17.6) Ctrough
(mcg/mL)LD 9 241 (52.1) 5 186 (16.5) 55 313 (33.9) MD 7 683 (46.1) 6 549 (34.1) 46 507 (42.5) Table 19: Mean (%CV) Pharmacokinetic Parameters Following ULTOMIRIS Treatment in Adult Patients with gMG N Adult Patients (ALXN1210-MG-306) Abbreviations: LD = Loading Dose; MD=Maintenance Dose Cmax
(mcg/mL)LD 86 874 (21.1) MD 76 1548 (23.2) Ctrough
(mcg/mL)LD 85 418 (27.6) MD 70 587 (29.6) Table 20: Mean (%CV) Pharmacokinetic Parameters Following ULTOMIRIS Treatment in Adult Patients with NMOSD N Adult Patients (ALXN1210-NMO-307) Abbreviations: LD = Loading Dose; MD=Maintenance Dose Cmax
(mcg/mL)LD 58 935.3 (17.3) MD 56 1836.4 (19.4) Ctrough
(mcg/mL)LD 58 459.1 (19.7) MD 54 796.9 (27.1) Distribution
The mean (standard deviation [SD]) volume of distribution at steady state in patients with PNH, aHUS, gMG, or NMOSD are shown in Table 21.
Elimination
The mean (standard deviation [SD]) terminal elimination half-life and clearance of ravulizumab-cwvz are shown in Table 21.
Table 21: Distribution, Biotransformation, and Elimination Parameters Following ULTOMIRIS Treatment Adult and Pediatric Patients with PNH Adult and Pediatric Patients with aHUS Adult Patients with gMG Adult Patients with NMOSD Distribution Volume of distribution at steady state (liters)
Mean (SD)5.30 (0.95) 5.22 (1.85) 5.74 (1.16) 4.77 (0.819) Biotransformation and Elimination Terminal elimination half-life (days)
Mean (SD)49.6 (9.08) 51.8 (16.2) 56.6 (8.36) 64.3 (11.0) Clearance (liters/day)
Mean (SD)0.08 (0.02) 0.08 (0.04) 0.08 (0.02) 0.05 (0.016) Specific Populations
No clinically significant differences in the pharmacokinetics of ravulizumab-cwvz were observed based on sex, age (10 months to 83 years), race, hepatic impairment, or any degree of renal impairment, including patients with proteinuria or receiving dialysis.
Body weight was a clinically significant covariate on the pharmacokinetics of ravulizumab-cwvz.
Drug Interactions
No drug-drug interaction studies have been performed.
Neonatal Fc Receptor blocker treatment may interfere with the endosomal neonatal FcRn recycling mechanism of monoclonal antibodies such as ravulizumab and thereby decrease serum ravulizumab concentrations [see Drug Interactions (7.1, 7.2)].
Concomitant PE, PP, or IVIg treatment requires a supplemental dose of ULTOMIRIS [see Dosage and Administration (2.5)].
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of ULTOMIRIS or other ravulizumab-cwvz products.
The immunogenicity of ravulizumab-cwvz has been evaluated using an enzyme-linked immunosorbent assay (ELISA) for the detection of binding anti-ravulizumab-cwvz antibodies. For patients whose sera tested positive in the screening immunoassay, an in vitro biological assay was performed to detect neutralizing antibodies.
In clinical studies with ULTOMIRIS, treatment-emergent antibodies to ravulizumab-cwvz were detected in 1 of 219 (0.5%) patients with PNH [see Clinical Studies (14.1)] and 1 of 71 (1.4%) patients with aHUS [see Clinical Studies (14.2)]. In these 2 patient populations, the observed ADA were non-neutralizing with no apparent impact on PK, safety, or efficacy. In the gMG study (N=86) and NMOSD study (N=58), no treatment-emergent antibodies to ravulizumab-cwvz were detected [see Clinical Studies (14.3 & 14.4)].
However, the assay used to measure anti-drug antibodies (ADA) is subject to interference by serum ravulizumab-cwvz, possibly resulting in an underestimation of the incidence of antibody formation. Due to the limitation of the assay conditions, the potential clinical impact of antibodies to ravulizumab-cwvz is not known.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal carcinogenicity studies of ravulizumab-cwvz have not been conducted.
Genotoxicity studies have not been conducted with ravulizumab-cwvz.
Effects of ravulizumab-cwvz upon fertility have not been studied in animals. Intravenous injections of male and female mice with a murine anti-C5 antibody at up to 0.8-2.2 times the equivalent of the clinical dose of ULTOMIRIS had no adverse effects on mating or fertility.
-
14 CLINICAL STUDIES
14.1 Paroxysmal Nocturnal Hemoglobinuria (PNH)
The safety and efficacy of ULTOMIRIS in adult patients with PNH was assessed in 2 open-label, randomized, active-controlled, non-inferiority Phase 3 studies: PNH Study 301 and PNH Study 302. Study 301 enrolled patients with PNH who were complement inhibitor naïve and had active hemolysis. Study 302 enrolled patients with PNH who were clinically stable after having been treated with eculizumab for at least the past 6 months. The safety and efficacy of ULTOMIRIS in pediatric patients with PNH was assessed in PNH Study 304, an open-label, Phase 3 study conducted in eculizumab-experienced and complement inhibitor treatment naïve pediatric patients with PNH.
In Study 301 and Study 302, adults with PNH were dosed with ULTOMIRIS administered intravenously in accordance with the weight-based dosing described in Section 2.3 (4 infusions of ULTOMIRIS over 26 weeks) above. Eculizumab was administered on Days 1, 8, 15, and 22, followed by maintenance treatment with 900 mg of eculizumab on Day 29 and every 2 weeks (q2w) thereafter for a total of 26 weeks of treatment, according to the approved dosing regimen of eculizumab which was the standard-of-care for PNH at the time of the studies.
Patients were vaccinated against meningococcal infection prior to or at the time of initiating treatment with ULTOMIRIS or eculizumab, or received prophylactic treatment with appropriate antibiotics until 2 weeks after vaccination. Prophylactic treatment with appropriate antibiotics beyond 2 weeks after vaccination was at the discretion of the provider.
Study in Complement-Inhibitor Naïve Adult Patients with PNH
The Complement-Inhibitor Naïve Study [ALXN1210-PNH-301; NCT02946463] was a 26-week, multicenter, open-label, randomized, active-controlled, non-inferiority Phase 3 study conducted in 246 patients naïve to complement inhibitor treatment prior to study entry.
Patients with PNH with flow cytometric confirmation of at least 5% PNH cells were randomized 1:1 to either intravenously administered ULTOMIRIS or eculizumab. The mean total PNH granulocyte clone size was 85%, the mean total PNH monocyte clone size was 88%, and the mean total PNH RBC clone size was 39%. Ninety-eight percent of patients had a documented PNH-associated condition diagnosed prior to enrollment on the trial: anemia (85%), hemoglobinuria (63%), history of aplastic anemia (32%), history of renal failure (12%), myelodysplastic syndrome (5%), pregnancy complications (3%), and other (16%). Major baseline characteristics were balanced between treatment groups. Table 22 provides the baseline characteristics for the patients enrolled in the complement-inhibitor naïve study.
Table 22: Baseline Characteristics in the Complement-Inhibitor Naïve Study Parameter Statistics ULTOMIRIS
(N=125)Eculizumab
(N=121)Abbreviations: LDH = lactate dehydrogenase; max = maximum; min = minimum; MAVE = major adverse vascular event; pRBC = packed red blood cell; SD = standard deviation Age (years) at first infusion in study Mean (SD)
Min, max44.8 (15.2)
18, 8346.2 (16.2)
18, 86Sex Male n (%) 65 (52.0) 69 (57.0) Race Asian n (%) 72 (57.6) 57 (47.1) White 43 (34.4) 51 (42.1) Black or African American 2 (1.6) 4 (3.3) American Indian or Alaska Native 1 (0.8) 1 (0.8) Other 4 (3.2) 4 (3.3) Not reported 3 (2.4) 4 (3.3) Pre-treatment LDH levels (U/L) Median
Min, max1513.5
(378.0, 3759.5)1445.0
(423.5, 3139.5)Units of pRBC/whole blood transfused within 12 months prior to first dose Median
Min, max6.0
(1, 44)6.0
(1, 32)Antithrombotic agents used within 28 days prior to first dose n (%) 22 (17.6) 22 (18.2) Patients with a history of MAVE n (%) 17 (13.6) 25 (20.7) Patients with a history of thrombosis n (%) 17 (13.6) 20 (16.5) Patients with concomitant anticoagulant treatment n (%) 23 (18.4) 28 (23.1) Efficacy was established based upon transfusion avoidance and hemolysis as directly measured by normalization of LDH levels. Transfusion avoidance was defined as patients who did not receive a transfusion and did not meet the protocol specified guidelines for transfusion from baseline up to Day 183. Supportive efficacy data included the percent change from baseline in LDH levels, the proportion of patients with breakthrough hemolysis defined as at least one new or worsening symptom or sign of intravascular hemolysis in the presence of elevated LDH ≥ 2 × ULN, after prior LDH reduction to < 1.5 × ULN on therapy and the proportion of patients with stabilized hemoglobin.
Non-inferiority of ULTOMIRIS to eculizumab was demonstrated across endpoints in the complement-inhibitor naïve treatment population described in Table 23 below.
Table 23: Efficacy Results in the Complement-Inhibitor Naïve Study ULTOMIRIS
(N=125)Eculizumab
(N=121)Statistic for Comparison Treatment Effect
(95% CI)For the transfusion avoidance endpoint, treatment differences (95% CIs) are based on estimated differences in percent with 95% CI. For the lactate dehydrogenase normalization endpoint, the adjusted prevalence within each treatment is displayed.
Abbreviations: LDH = lactate dehydrogenase; CI = confidence intervalTransfusion avoidance rate 73.6% 66.1% Difference in rate 6.8
(-4.66, 18.14)LDH normalization 53.6% 49.4% Odds ratio 1.19
(0.80, 1.77)LDH percent change -76.84% -76.02% Difference in % change from baseline -0.83
(-5.21, 3.56)Breakthrough hemolysis 4.0% 10.7% Difference in rate -6.7
(-14.21, 0.18)Hemoglobin stabilization 68.0% 64.5% Difference in rate 2.9
(-8.80, 14.64)There was no observable difference in fatigue between ULTOMIRIS and eculizumab after 26 weeks of treatment compared to baseline as measured by the FACIT-fatigue instrument. Patient-reported fatigue may be an under- or over-estimation because patients were not blinded to treatment assignment.
Study in Eculizumab-Experienced Adult Patients with PNH
The study in eculizumab-experienced patients [ALXN1210-PNH-302; NCT03056040] was a 26-week, multicenter, open-label, randomized, active-controlled, non-inferiority Phase 3 study conducted in 195 patients with PNH who were clinically stable after having been treated with eculizumab for at least the past 6 months.
Patients who demonstrated clinically stable disease after being treated with eculizumab for at least the prior 6 months were randomized 1:1 to either continue eculizumab or to switch to ULTOMIRIS administered intravenously. The mean total PNH granulocyte clone size was 83%, the mean total PNH monocyte clone size was 86%, and the mean total PNH RBC clone size was 60%. Ninety-five percent of patients had a documented PNH-associated condition diagnosed prior to enrollment in the trial: anemia (67%), hematuria or hemoglobinuria (49%), history of aplastic anemia (37%), history of renal failure (9%), myelodysplastic syndrome (5%), pregnancy complication (7%), and other (14%). Major baseline characteristics were balanced between the 2 treatment groups. Table 24 provides the baseline characteristics for the patients enrolled in the eculizumab-experienced study.
Table 24: Baseline Characteristics in Eculizumab-Experienced Adult Patients with PNH Parameter Statistics ULTOMIRIS
(N=97)Eculizumab
(N=98)Abbreviations: LDH = lactate dehydrogenase; max = maximum; min = minimum; MAVE = major adverse vascular event; pRBC = packed red blood cell; SD = standard deviation Age (years) at first infusion in study Mean (SD)
Min, max46.6 (14.41)
18, 7948.8 (13.97)
23, 77Race n (%) White 50 (51.5) 61 (62.2) Asian 23 (23.7) 19 (19.4) Black or African American 5 (5.2) 3 (3.1) Other 2 (2.1) 1 (1.0) Not reported 13 (13.4) 13 (13.3) Unknown 3 (3.1) 1 (1.0) Multiple 1 (1.0) 0 Sex n (%) Male 50 (51.5) 48 (49.0) Pre-treatment LDH levels (U/L) Median
Min, max224.0
135.0, 383.5234.0
100.0, 365.5Units of pRBC/whole blood transfused within 12 months prior to first dose Median
Min, max4.0
(1, 32)2.5
(2, 15)Antithrombotic agents used within 28 days prior to first dose n (%) 20 (20.6) 13 (13.3) Patients with a history of MAVE n (%) 28 (28.9) 22 (22.4) Patients with a history of thrombosis n (%) 27 (27.8) 21 (21.4) Patients with concomitant anticoagulant treatment n (%) 22 (22.7) 16 (16.3) Efficacy was established based on hemolysis as measured by LDH percent change from baseline to Day 183 and supportive efficacy data was transfusion avoidance, proportion of patients with stabilized hemoglobin, and the proportion of patients with breakthrough hemolysis through Day 183.
Non-inferiority of ULTOMIRIS to eculizumab was demonstrated across endpoints in the patients with PNH previously treated with eculizumab described in Table 25 below.
Table 25: Efficacy Results in the Eculizumab-Experienced Adult Patients with PNH Eculizumab-Experienced Study ULTOMIRIS
N = 97Eculizumab
N = 98Statistic for Comparison Treatment Effect
(95% CI)Abbreviations: CI = confidence interval; LDH = lactate dehydrogenase LDH percent change -0.82% 8.4% Difference in % change from baseline 9.2
(-0.42, 18.8)Breakthrough hemolysis 0% 5.1% Difference in rate 5.1
(-8.9, 19.0)Transfusion avoidance 87.6 % 82.7% Difference in rate 5.5
(-4.3, 15.7)Hemoglobin stabilization 76.3% 75.5% Difference in rate 1.4
(-10.4, 13.3)There was no observable difference in fatigue between ULTOMIRIS and eculizumab after 26 weeks of treatment compared to baseline as measured by the FACIT-fatigue instrument. Patient-reported fatigue may be an under-or over-estimation because patients were not blinded to treatment assignment.
Study in Eculizumab-Experienced and Complement-Inhibitor Naïve Pediatric Patients with PNH
The pediatric study, ALXN1210-PNH-304 (NCT03406507), was a multi-center, open-label Phase 3 study conducted in eculizumab-experienced and complement inhibitor treatment-naïve pediatric patients with PNH. A total of 13 pediatric patients with PNH completed intravenously administered ULTOMIRIS treatment during the Primary Evaluation Period (26 weeks). Five of the 13 patients had never been treated with complement inhibitors and 8 patients were treated with eculizumab. Eleven of the thirteen patients were between 12 and 17 years of age at first infusion, with 2 patients under 12 years old (11 and 9 years old). Table 26 presents the baseline characteristics of the pediatric patients enrolled in Study ALXN1210-PNH-304.
Table 26: Baseline Characteristics for Pediatric Patients with PNH Variable Complement Inhibitor Treatment-naïve Patients
(N = 5)Eculizumab-Experienced Patients
(N = 8)All Patients
(N = 13)Note: Percentages were based on the total number of patients in each cohort, or overall.
Abbreviations: LDH = lactate dehydrogenase; kg = kilogram; max = maximum; min = minimum; pRBC = packed red blood cells; SD = standard deviationSex, n (%) Male 4 (80.0) 1 (12.5) 5 (38.5) Female 1 (20.0) 7 (87.5) 8 (61.5) Age at first infusion (years) Mean (SD) 14.4 (2.2) 14.4 (3.1) 14.4 (2.7) Median (min, max) 15.0 (11, 17) 15.0 (9, 17) 15.0 (9, 17) Age at first infusion (years) category, n (%) < 12 years 1 (20.0) 1 (12.5) 2 (15.4) ≥ 12 years 4 (80.0) 7 (87.5) 11 (84.6) Baseline weight (kg) Mean (SD) 56.3 (11.6) 56.3 (12.2) 56.3 (11.5) Median (min, max) 55.6 (39.5, 72.0) 55.5 (36.7, 69.0) 55.6 (36.7, 72.0) Baseline weight (kg) category, n (%) ≥ 30 to < 40 kg 1 (20.0) 1 (12.5) 2 (15.4) ≥ 40 to < 60 kg 3 (60.0) 4 (50.0) 7 (53.8) ≥ 60 to < 100 kg 1 (20.0) 3 (37.5) 4 (30.8) Units of pRBC/whole blood transfused within 12 months prior to first dose Median (min, max) 7.0 (3, 11) 2.0 (2, 2) - Pre-treatment LDH levels (U/L) Median (min, max) 588.5 (444, 2269.7) 251.5 (140.5, 487) - Based on body weight, patients received a loading dose of ULTOMIRIS on Day 1, followed by maintenance treatment on Day 15 and once every 8 weeks (q8w) thereafter for patients weighing ≥ 20 kg, or once every 4 weeks (q4w) for patients weighing < 20 kg. For patients who entered the study on eculizumab therapy, Day 1 of study treatment was planned to occur 2 weeks from the patient's last dose of eculizumab.
The weight-based dose regimen of ravulizumab-cwvz provided inhibition of terminal complement in all patients throughout the entire 26-week treatment period regardless of prior experience with eculizumab. Following initiation of ravulizumab-cwvz treatment, steady-state therapeutic serum concentrations of ravulizumab-cwvz were achieved after the first dose and maintained throughout the primary evaluation period in both cohorts. Three of 5 complement inhibitor treatment-naïve patients and 6 out of 8 eculizumab-experienced patients achieved hemoglobin stabilization by Week 26, respectively. Transfusion avoidance was reached for 11 out of 13 of patients during the 26-week Primary Evaluation Period. One patient experienced breakthrough hemolysis during the extension period. Table 27 presents secondary efficacy outcomes for the primary evaluation period.
Table 27: Efficacy Outcomes from the 26-Week Primary Evaluation Period of Pediatric Patient Study in PNH (ALXN1210-PNH-304) Endpoint Treatment Naïve
(N = 5)Eculizumab Experienced
(N = 8)Abbreviations: FACIT = Functional Assessment of Chronic Illness Therapy; LDH = lactate dehydrogenase - *
- 95% CIs for the mean obtained from t-distribution were presented.
- †
- 95% CIs for the proportion were based on exact confidence limits using the Clopper-Pearson method.
- ‡
- No patients experienced breakthrough hemolysis during the primary evaluation period. One patient experienced breakthrough hemolysis at 1.8 years during the extension period; however, at the time of the breakthrough hemolysis event the patient had adequate C5 inhibition (free C5 < 0.5 mcg/mL).
LDH percent change from baseline (%)* -47.9 (-113.4, 17.5) 4.7 (-36.7, 46.0) Transfusion avoidance (%)† 60.0 (14.7, 94.7) 100.0 (63.1, 100.0) Change in FACIT-Fatigue* 3.4 (-4.2, 11.0) 1.3 (-3.1, 5.7) Hemoglobin stabilization (%)† 60.0 (14.7, 94.7) 75.0 (34.9, 96.8) Breakthrough hemolysis (%) 0 0‡ A clinically relevant improvement from baseline in fatigue as assessed by Pediatric FACIT-Fatigue (i.e., mean improvement of > 3 units for Pediatric FACIT Fatigue scores) was sustained throughout the primary evaluation period in the 5-complement inhibitor treatment naïve patients. A slight improvement was also observed in eculizumab-experienced patients. However, patient-reported fatigue may be an under- or over-estimation because patients were not blinded to treatment assignment.
The efficacy of ULTOMIRIS in pediatric patients with PNH is similar to that observed in adult patients with PNH enrolled in pivotal studies.
14.2 Atypical Hemolytic Uremic Syndrome (aHUS)
The efficacy of ULTOMIRIS administered intravenously in patients with aHUS was assessed in 2 open-label, single-arm studies. Study ALXN1210-aHUS-311 enrolled adult patients who displayed signs of TMA. In order to qualify for enrollment, patients were required to have a platelet count ≤ 150 × 109/L, evidence of hemolysis such as an elevation in serum LDH, and serum creatinine above the upper limits of normal or required dialysis.
Study ALXN1210-aHUS-312 enrolled pediatric patients who displayed signs of TMA. In order to qualify for enrollment, patients were required to have a platelet count ≤ 150 × 109/L, evidence of hemolysis such as an elevation in serum LDH, and serum creatinine level ≥ 97.5% percentile at screening or required dialysis. In both studies, enrollment criteria excluded patients presenting with TMA due to a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) deficiency, Shiga toxin Escherichia coli related hemolytic uremic syndrome (STEC-HUS) and genetic defect in cobalamin C metabolism. Patients with confirmed diagnosis of STEC-HUS after enrollment were excluded from the efficacy evaluation.
Study in Adult Patients with aHUS
The adult study [ALXN1210-aHUS-311; NCT02949128] was conducted in patients who were naïve to complement inhibitor treatment prior to study entry. The study consisted of a 26-week Initial Evaluation Period and patients were allowed to enter an extension period for up to 4.5 years. All patients received ULTOMIRIS administered intravenously according to their weight [see Dosage and Administration (2.3)].
A total of 56 patients with aHUS were evaluated for efficacy. Ninety-three percent of patients had extra-renal signs (cardiovascular, pulmonary, central nervous system, gastrointestinal, skin, skeletal muscle) or symptoms of aHUS at baseline. At baseline, 71.4% (n = 40) of patients had Stage 5 chronic kidney disease (CKD). Fourteen percent had a medical history of kidney transplant and 51.8% were on dialysis at study entry. Eight patients entered the study with evidence of TMA for > 3 days after childbirth (i.e., postpartum).
Table 28 presents the demographics and baseline characteristics of the 56 adult patients enrolled in Study ALXN1210-aHUS-311 that constituted the Full Analysis Set.
Table 28: Demographics and Baseline Characteristics in Study ALXN1210-aHUS-311 Parameter Statistics ULTOMIRIS
(N=56)Note: Percentages are based on the total number of patients.
Abbreviations: eGFR = estimated glomerular filtration rate; LDH = lactate dehydrogenase; max = maximum; min = minimum; SD = standard deviation- *
- Patients can have multiple races selected.
Age at time of first infusion (years)
Mean (SD)
Min, max42.2 (14.98)
19.5, 76.6Sex Female n (%) 37 (66.1) Race * n (%) White 29 (51.8) Asian 15 (26.8) Unknown 8 (14.3) Other 4 (7.1) Platelets (109/L) blood
[normal range 130 to 400 × 109/L]n
Median (min,max)56
95.25 (18, 473)Hemoglobin (g/L) blood
[normal range 115 to 160 g/L (female), 130 to 175 g/L (male)]n
Median (min,max)56
85.00 (60.5, 140)LDH (U/L) serum
[normal range 120 to 246 U/L]n
Median (min,max)56
508.00 (229.5, 3249)eGFR (mL/min/1.73 m2)
[normal range ≥ 60 mL/min/1.73 m2]n (%)
Mean (SD)
Median (min,max)55
15.86 (14.815)
10.00 (4, 80)The efficacy evaluation was based on Complete TMA Response during the 26-week Initial Evaluation Period, as evidenced by normalization of hematological parameters (platelet count and LDH) and ≥ 25% improvement in serum creatinine from baseline. Patients had to meet each Complete TMA Response criteria at 2 separate assessments obtained at least 4 weeks (28 days) apart, and any measurement in between.
Complete TMA Response was observed in 30 of the 56 patients (54%) during the 26-week Initial Evaluation Period as shown in Table 29.
Table 29: Efficacy Results in aHUS during the 26-Week Initial Evaluation Period (ALXN1210-aHUS-311) Total Responder n Proportion (95% CI)* Abbreviations: CI = confidence interval; LDH = lactate dehydrogenase; TMA = thrombotic microangiopathy - *
- 95% CIs for the proportion were based on exact confidence limits using the Clopper-Pearson method.
Complete TMA Response 56 30 0.54 (0.40, 0.67) Components of Complete TMA Response Platelet count normalization 56 47 0.84 (0.72, 0.92) LDH normalization 56 43 0.77 (0.64, 0.87) ≥ 25% improvement in serum creatinine from baseline 56 33 0.59 (0.45, 0.72) Hematologic normalization 56 41 0.73 (0.60, 0.84) One additional patient had a Complete TMA Response that was confirmed after the 26-week Initial Evaluation Period. Complete TMA Response was achieved at a median time of 86 days (range: 7 to 169 days). The median duration of Complete TMA Response was 7.97 months (range: 2.52 to 16.69 months). All responses were maintained through all available follow-up.
Other endpoints included platelet count change from baseline, dialysis requirement, and renal function as evaluated by estimated glomerular filtration rate (eGFR).
An increase in mean platelet count was observed after commencement of ULTOMIRIS treatment, increasing from 118.52 × 109/L at baseline to 240.34 ×109/L at Day 8 and remaining above 227 × 109/L at all subsequent visits in the Initial Evaluation Period (26 weeks).
Renal function, as measured by eGFR, was improved or maintained during ULTOMIRIS therapy. The mean eGFR (+/- SD) increased from 15.86 (14.82) at baseline to 51.83 (39.16) by 26 weeks. In patients with Complete TMA Response, renal function continued to improve after the Complete TMA Response was achieved.
Seventeen of the 29 patients (59%) who required dialysis at study entry discontinued dialysis by the end of the available follow-up and 6 of 27 (22%) patients were off dialysis at baseline were on dialysis at last available follow-up.
Study in Pediatric Patients with aHUS
The Pediatric Study [ALXN1210-aHUS-312; NCT03131219] is a 26-week ongoing, multicenter, single-arm study conducted in 16 pediatric patients. All patients received ULTOMIRIS administered intravenously according to their weight [see Dosage and Administration (2.3)]
A total of 14 eculizumab-naïve patients with documented diagnosis of aHUS were enrolled and included in this interim analysis. The median age at the time of first infusion was 5.2 years (range 0.9, 17.3 years). The overall mean weight at Baseline was 19.8 kg; half of the patients were in the baseline weight category ≥ 10 to < 20 kg. The majority of patients (71%) had pretreatment extra-renal signs (cardiovascular, pulmonary, central nervous system, gastrointestinal, skin, skeletal muscle) or symptoms of aHUS at baseline. At baseline, 35.7% (n = 5) of patients had a CKD Stage 5. Seven percent had history of prior kidney transplant and 35.7% were on dialysis at study entry.
Table 30 presents the baseline characteristics of the pediatric patients enrolled in Study ALXN1210-aHUS-312.
Table 30: Demographics and Baseline Characteristics in Study ALXN1210-aHUS-312 Parameter Statistics ULTOMIRIS
(N = 14)Note: Percentages are based on the total number of patients.
Abbreviations: eGFR = estimated glomerular filtration rate; LDH = lactate dehydrogenase; max = maximum; min = minimum- *
- Patients can have multiple races selected.
Age at time of first infusion (years) category n (%) Birth to < 2 years 2 (14.3) 2 to < 6 years 7 (50.0) 6 to < 12 years 4 (28.6) 12 to < 18 years 1 (7.1) Sex n (%) Female 9 (64.3) Race* n (%) White 7 (50.0) Asian 4 (28.6) Black or African American 2 (14.3) American Indian or Alaskan Native 1 (7.1) Unknown 1 (7.1) Platelets (109/L) blood [normal range 229 to 533 × 109/L] Median (min, max) 64.00 (14, 125) Hemoglobin (g/L) blood [normal range 107 to 131 g/L] Median (min, max) 74.25 (32, 106) LDH (U/L) serum [normal range 165 to 395 U/L] Median (min, max) 2077.00 (772, 4985) eGFR (mL/min/1.73 m2) [normal range ≥ 60 mL/min/1.73 m2] Mean (SD)
Median (min, max)28.4 (23.11)
22.0 (10, 84)Efficacy evaluation was based upon Complete TMA Response during the 26-week Initial Evaluation Period, as evidenced by normalization of hematological parameters (platelet count and LDH) and ≥ 25% improvement in serum creatinine from baseline. Patients had to meet all Complete TMA Response criteria at 2 separate assessments obtained at least 4 weeks (28 days) apart, and any measurement in between.
Complete TMA Response was observed in 10 of the 14 patients (71%) during the 26-week Initial Evaluation Period as shown in Table 31.
Table 31: Efficacy Results in aHUS during the 26-Week Initial Evaluation Period (ALXN1210-aHUS-312) Total Responder n Proportion (95% CI)* Note: One patient withdrew from study after receiving 2 doses of ravulizumab-cwvz.
Abbreviations: CI = confidence interval; LDH = lactate dehydrogenase; TMA = thrombotic microangiopathy- *
- 95% CIs for the proportion were based on exact confidence limits using the Clopper-Pearson method.
Complete TMA Response 14 10 0.71 (0.42, 0.92) Components of Complete TMA Response Platelet count normalization 14 13 0.93 (0.66, 0.99) LDH normalization 14 12 0.86 (0.57, 0.98) ≥ 25% improvement in serum creatinine from baseline 14 11 0.79 (0.49, 0.95) Hematologic normalization 14 12 0.86 (0.57, 0.98) Complete TMA Response during the Initial Evaluation Period was achieved at a median time of 30 days (range:15 to 88 days). The median duration of Complete TMA Response was 5.08 months (range: 3.08 to 5.54 months). All responses were maintained through all available follow-up.
Other endpoints included platelet count change from baseline, dialysis requirement, and renal function as evaluated by eGFR.
An increase in mean platelet count was observed after commencement of ULTOMIRIS treatment, increasing from 60.50 × 109/L at baseline to 296.67 × 109/L at Day 8 and remained above 296 × 109/L at all subsequent visits in the Initial Evaluation Period (26 weeks). The mean eGFR (+/- SD) increased from 28.4 (23.11) at baseline to 108.0 (63.21) by 26 weeks.
Four of the 5 patients who required dialysis at study entry were able to discontinue dialysis after the first month in study and for the duration of ULTOMIRIS treatment. No patient started dialysis during the study.
14.3 Generalized Myasthenia Gravis (gMG)
The efficacy of ULTOMIRIS for the treatment of gMG was demonstrated in a randomized, double-blind, placebo-controlled, multicenter study (ALXN1210-MG-306; NCT03920293). Patients were randomized 1:1 to either receive ULTOMIRIS (n=86) or placebo (n=89) for 26 weeks. ULTOMIRIS was administered intravenously according to the weight-based recommended dosage [see Dosage and Administration (2.3)].
Patients with gMG with a positive serologic test for anti-AChR antibodies, Myasthenia Gravis Foundation of America (MGFA) clinical classification class II to IV, and Myasthenia Gravis-Activities of Daily Living (MG-ADL) total score ≥ 6 were enrolled. Baseline and disease characteristics were similar between treatment groups (including age at first dose [mean of 58 years for ULTOMIRIS versus 53 years for placebo], gender [51% female for ULTOMIRIS versus 51% female for placebo], race as White, Asian, and Black or African American [78%, 17%, and 2% for ULTOMIRIS versus 69%, 18%, and 5% for placebo, respectively], and duration of MG since diagnosis [mean of 10 years, ranging from 0.5 to 39.5 years, for ULTOMIRIS versus mean of 10.0 years, ranging from 0.5 to 36.1 years, for placebo].
Over 80% of patients were receiving acetylcholinesterase inhibitors, 70% were receiving corticosteroids, and 68% were receiving non-steroidal immunosuppressants (ISTs) at study entry. Patients on concomitant medications to treat gMG were permitted to continue on therapy throughout the course of the study.
The primary efficacy endpoint was a comparison of the change from baseline between treatment groups in the MG-ADL total score at Week 26. The MG-ADL is a categorical scale that assesses the impact on daily function of 8 signs or symptoms that are typically affected in gMG. Each item is assessed on a 4-point scale where a score of 0 represents normal function and a score of 3 represents loss of ability to perform that function. The total score ranges from 0 to 24, with the higher scores indicating more impairment.
The secondary endpoints, also assessed from baseline to Week 26, included the change in the Quantitative MG total score (QMG). The QMG is a 13-item categorical scale assessing muscle weakness. Each item is assessed on a 4-point scale where a score of 0 represents no weakness and a score of 3 represents severe weakness. A total score ranges from 0 to 39, where higher scores indicate more severe impairment.
Other secondary endpoints included the proportion of patients with improvements of at least 5 and 3 points in the QMG and MG-ADL total scores, respectively.
Treatment with ULTOMIRIS demonstrated a statistically significant change in the MG-ADL and QMG total scores from baseline at Week 26 as compared to placebo (Table 32).
Table 32: Efficacy Results in Patients with gMG Efficacy Endpoints: Change from Baseline at Week 26 Placebo
(n = 89)
LS MeanULTOMIRIS
(n = 86)
LS MeanTreatment Effect
(95% CI)p-value* Abbreviations: CI = confidence interval, LS = least squares; MG-ADL = Myasthenia Gravis Activities of Daily Living profile; QMG = Quantitative Myasthenia Gravis score for disease severity - *
- p-value calculated using mixed effect model for repeated measures
Primary Endpoint MG-ADL -1.4 -3.1 -1.6 (-2.6, -0.7) < 0.001 Secondary Endpoint QMG -0.8 -2.8 -2.0 (-3.2, -0.8) < 0.001 The proportion of QMG responders with at least a 5-point improvement at week 26 was greater for ULTOMIRIS (30.0%) compared to placebo (11.3%) p = 0.005. The proportion of MG-ADL responders with at least a 3-point improvement at week 26 was also greater for ULTOMIRIS (56.7%) compared to placebo (34.1%). The proportion of clinical responders at higher response thresholds (≥ 4-, 5-, 6-, 7-, or 8-point improvement on MG-ADL, and ≥ 6-, 7-, 8-, 9-, or 10-point improvement on QMG) was consistently greater for ULTOMIRIS compared to placebo.
Figure 1: Change from Baseline in MG-ADL Total Score (A) and QMG Total Score (B) Through Week 26 of the Randomized Controlled Period of ALXN1210-MG-306 (Mean and 95% CI)
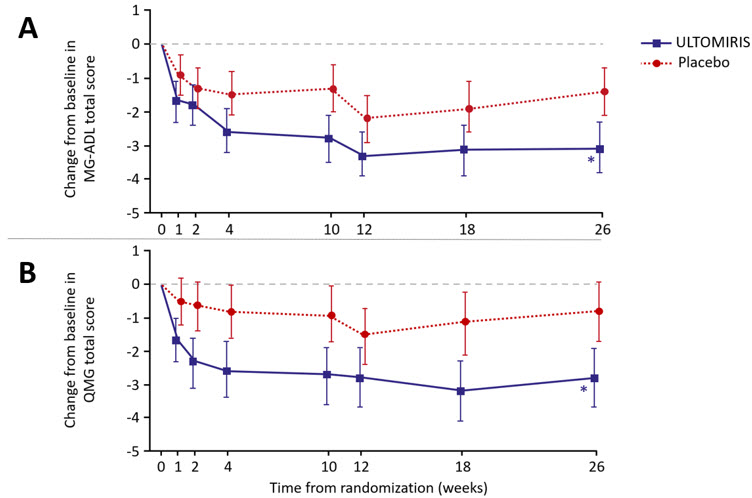
Note: *p<0.001 versus placebo
14.4 Neuromyelitis Optica Spectrum Disorder (NMOSD)
The efficacy and safety of ULTOMIRIS in adult patients with anti-AQP4 antibody positive NMOSD was assessed in an open-label multicenter study, Study ALXN1210-NMO-307 (NCT04291262). Patients participating in Study ALXN1210-NMO-307 received ULTOMIRIS intravenously in the Primary Treatment Period that ended when the last enrolled patient completed (or discontinued prior to) 50 weeks on study, representing a median study duration of 73.5 weeks (minimum 13.7, maximum 117.7). Efficacy assessments were based on a comparison of patients in Study ALXN1210-NMO-307 with an external placebo control group from another study (Study ECU-NMO-301, NCT01892345) composed of a comparable population of adult patients with anti-AQP4 antibody positive NMOSD.
Study ALXN1210-NMO-307 enrolled 58 adult patients with NMOSD who had a positive serologic test for anti-AQP4 antibodies, at least 1 relapse in the last 12 months prior to the Screening Period, and an Expanded Disability Status Scale (EDSS) score ≤ 7. In the external placebo control group, eligibility criteria were similar except patients were required to have at least 2 relapses in last 12 months or 3 relapses in the last 24 months with at least 1 relapse in the 12 months prior to screening. Prior treatment with immunosuppressant therapies (ISTs) was not required for enrollment. However, patients on selected ISTs (i.e., corticosteroids, azathioprine, mycophenolate mofetil, methotrexate, and tacrolimus) were permitted to continue on therapy, with a requirement for stable dosing until they reached Week 106 in the Study. Similar IST use was permitted in the external placebo control group.
ULTOMIRIS was administered intravenously according to the weight-based recommended dosage [see Dosage and Administration (2.3)].
The demographics were similar between the ULTOMIRIS treatment group from Study ALXN1210-NMO-307 and the placebo treatment group from Study ECU-NMO-301 (including age [median of 46.0 years for ULTOMIRIS versus 44.0 years for placebo] and sex [89.7% female for ULTOMIRIS versus 89.4% female for placebo]). The majority of patients were White or Asian. The median time from diagnosis to first dose was 0.9 years for ULTOMIRIS and 2.0 years for placebo. The median annualized relapse rate (ARR) in the last 24 months was 1.4 for ULTOMIRIS versus 1.9 for placebo, and the median number of historical relapses was 2 for ULTOMIRIS versus 4 for placebo. The median baseline EDSS score was 3.3 for ULTOMIRIS versus 4.0 for placebo. At baseline, 48% of patients in the ULTOMIRIS group received concomitant IST, including corticosteroids, versus 72% of subjects in the placebo group.
The primary endpoint of Study ALXN1210-NMO-307 was the time to first adjudicated on-trial relapse as determined by an independent adjudication committee. No adjudicated on-trial relapses were observed in ULTOMIRIS-treated patients during the Primary Treatment Period, representing a statistically significant difference between the ULTOMIRIS and placebo treatment arms in time to first adjudicated on-trial relapse (p < 0.0001). The hazard ratio (95% confidence interval [CI]) for ULTOMIRIS compared with placebo was 0.014 (0.000, 0.103), representing a 98.6% reduction in the risk of relapse (Figure 2). ULTOMIRIS-treated patients experienced similar improvement in time to first adjudicated on-trial relapse with or without concomitant treatment.
Figure 2: Kaplan-Meier Survival Estimates for Time to First Adjudicated On-Trial Relapse in Study ALXN1210-NMO-307 and Comparative Placebo Arm of Study ECU-NMO-301
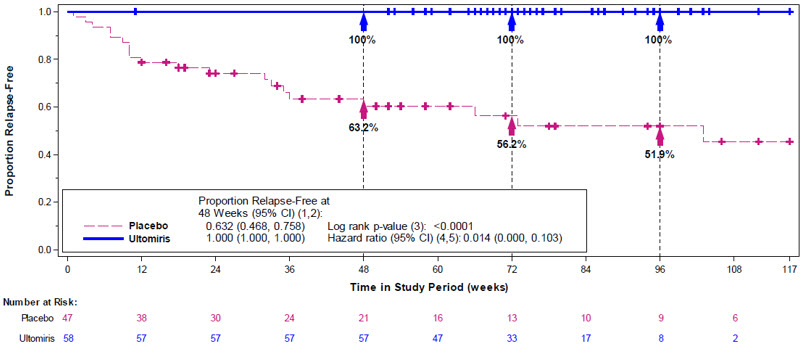
Note: The placebo group data were collected as part of Study ECU-NMO-301. Patients who did not experience an adjudicated on-trial relapse were censored at the end of the study period. If a patient in the placebo group was followed longer than any of the patients in the Ultomiris group, then that patient was censored at the longest Ultomiris follow-up time.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
ULTOMIRIS (ravulizumab-cwvz) injection 100 mg/mL is translucent, clear to yellowish color solution supplied in one single-dose vial per carton as:
- 300 mg/3 mL (100 mg/mL) (NDC 25682-025-01)
- 1,100 mg/11 mL (100 mg/mL) (NDC 25682-028-01)
ULTOMIRIS (ravulizumab-cwvz) injection 10 mg/mL is clear to translucent, slight whitish color solution supplied in one single-dose vial per carton as:
- 300 mg/30 mL (10 mg/mL) (NDC 25682-022-01)
-
17 PATIENT COUNSELING INFORMATION
Advise the patients and/or caregivers to read the FDA-approved patient labeling (Medication Guide).
Serious Meningococcal Infections
Advise patients of the risk of serious meningococcal infection. Inform patients of the need to complete or update their meningococcal vaccinations at least 2 weeks prior to receiving the first dose of ULTOMIRIS or receive antibacterial drug prophylaxis if ULTOMIRIS treatment must be initiated immediately and they have not been previously vaccinated. Inform patients of the requirement to be revaccinated according to current ACIP recommendations for meningococcal infection while on ULTOMIRIS therapy [see Warnings and Precautions (5.1)].
Inform patients that vaccination may not prevent serious meningococcal infection and to seek immediate medical attention if the following signs or symptoms occur [see Warnings and Precautions (5.1)]:
- fever
- fever and a rash
- headache with nausea or vomiting
- fever with high heart rate
- headache and a fever
- headache with a stiff neck or stiff back
- confusion
- muscle aches with flu-like symptoms
- eyes sensitive to light
Inform patients that they will be given a Patient Safety Card for ULTOMIRIS that they should carry with them at all times during and for 8 months following treatment with ULTOMIRIS. This card describes symptoms which, if experienced, should prompt the patient to immediately seek medical evaluation.
ULTOMIRIS and SOLIRIS REMS
ULTOMIRIS is available only through a restricted program called ULTOMIRIS and SOLIRIS REMS [see Warnings and Precautions (5.2)].
Inform the patient of the following notable requirements:
- Patients must receive counseling about the risk of serious meningococcal infections.
- Patients must receive written educational materials about this risk.
- Patients must be instructed to carry the Patient Safety Card with them at all times during and for 8 months following treatment with ULTOMIRIS.
- Patients must be instructed to complete or update meningococcal vaccines for serogroups A, C, W, Y and B per ACIP recommendations as directed by the prescriber prior to treatment with ULTOMIRIS.
- Patients must receive antibiotics as directed by the prescriber if they are not up to date with meningococcal vaccines and have to start ULTOMIRIS right away.
Other Infections
Counsel patients of the increased risk of infections, particularly those due to encapsulated bacteria, especially Neisseria species. Advise patients of the need for vaccination against meningococcal infections according to current medical guidelines. Counsel patients about gonorrhea prevention and advise regular testing for patients at risk. Advise patients to report any new signs and symptoms of infection.
Discontinuation
Inform patients with PNH or aHUS that they may develop serious hemolysis or TMA, respectively, when ULTOMIRIS is discontinued and that they will be monitored by their healthcare professional for at least 16 weeks for PNH or at least 12 months for aHUS following ULTOMIRIS discontinuation [see Warnings and Precautions (5.4)].
Inform patients who discontinue ULTOMIRIS to keep the Patient Safety Card with them for 8 months after the last ULTOMIRIS dose, because the increased risk of meningococcal infection persists for several months following discontinuation of ULTOMIRIS.
Infusion-Related Reactions
Advise patients that administration of ULTOMIRIS may result in infusion-related reactions [see Warnings and Precautions (5.6)].
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ULTOMIRIS during pregnancy. Encourage participation and advise patients about how they may enroll in the registry [see Use in Specific Populations (8.1)].
-
SPL UNCLASSIFIED SECTION
Manufactured by:
Alexion Pharmaceuticals, Inc.
121 Seaport Boulevard
Boston, MA 02210 USAUS License Number 1743
This product, or its use, may be covered by one or more US patents, including US Patent No. 9,079,949; 9,107,861; 9,206,251; 9,371,377; 9,663,574; 9,803,007; 10,227,400; 10,584,164; and 11,365,241 in addition to others including patents pending.
ULTOMIRIS is a registered trademark of Alexion Pharmaceuticals, Inc.
© 2024 Alexion Pharmaceuticals, Inc.
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 06/2024 MEDICATION GUIDE
ULTOMIRIS® (ul-toe-meer-is)
(ravulizumab-cwvz)
injection, for intravenous useWhat is the most important information I should know about ULTOMIRIS?
ULTOMIRIS is a medicine that affects your immune system. ULTOMIRIS may lower the ability of your immune system to fight infections.-
ULTOMIRIS increases your chance of getting serious meningococcal infections caused by Neisseria meningitidis bacteria. Meningococcal infections may quickly become life-threatening or cause death if not recognized and treated early.
- You must complete or update your meningococcal vaccine(s) at least 2 weeks before your first dose of ULTOMIRIS.
- If you have not completed your meningococcal vaccines and ULTOMIRIS must be started right away, you should receive the required vaccine(s) as soon as possible.
- If you have not been vaccinated and ULTOMIRIS must be started right away, you should also receive antibiotics to take for as long as your healthcare provider tells you.
- If you had a meningococcal vaccine in the past, you might need additional vaccines before starting ULTOMIRIS. Your healthcare provider will decide if you need additional meningococcal vaccines.
- Meningococcal vaccines do not prevent all meningococcal infections. Call your healthcare provider or get emergency medical care right away if you get any of these signs and symptoms of a serious meningococcal infection:
- fever
- fever with high heart rate
- headache and fever
- confusion
- muscle aches with flu-like symptoms
- fever and a rash
- headache with nausea or vomiting
- headache with stiff neck or stiff back
- eyes sensitive to light
Your healthcare provider will give you a Patient Safety Card about the risk of serious meningococcal infection. Carry it with you at all times during treatment and for 8 months after your last dose of ULTOMIRIS. Your risk of meningococcal infection may continue for several months after your last dose of ULTOMIRIS. It is important to show this card to any healthcare provider who treats you. This will help them diagnose and treat you quickly. ULTOMIRIS is only available through a program called the ULTOMIRIS and SOLIRIS Risk Evaluation and Mitigation Strategy (REMS). Before you can receive ULTOMIRIS, your healthcare provider must: - enroll in the ULTOMIRIS and SOLIRIS REMS program
- counsel you about the risk of serious meningococcal infections
- give you information about the signs and symptoms of serious meningococcal infection
- make sure that you are vaccinated against serious infections caused by meningococcal bacteria and that you receive antibiotics if you need to start ULTOMIRIS right away and you are not up to date on your vaccines
- give you a Patient Safety Card about your risk of meningococcal infection, as discussed above
ULTOMIRIS may also increase the risk of other types of serious infections caused by encapsulated bacteria, including Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria gonorrhoeae. - If your child is treated with ULTOMIRIS, your child should receive vaccines against Streptococcus pneumoniae and Haemophilus influenzae type b (Hib).
- Certain people may be at risk of serious infections with gonorrhea. Talk to your healthcare provider about whether you are at risk for gonorrhea infection, about gonorrhea prevention, and regular testing.
For more information about side effects, see "What are the possible side effects of ULTOMIRIS?" What is ULTOMIRIS? ULTOMIRIS is a prescription medicine called a monoclonal antibody. ULTOMIRIS is used to treat: - adults and children 1 month of age and older with a disease called Paroxysmal Nocturnal Hemoglobinuria (PNH).
- adults and children 1 month of age and older with a disease called atypical Hemolytic Uremic Syndrome (aHUS).
ULTOMIRIS is not used in treating people with Shiga toxin E. coli related hemolytic uremic syndrome (STEC-HUS). - adults with a disease called generalized Myasthenia Gravis (gMG) who are anti-acetylcholine receptor (AChR) antibody positive.
- adults with a disease called Neuromyelitis Optica Spectrum Disorder (NMOSD) who are anti-aquaporin-4 (AQP4) antibody positive.
It is not known if ULTOMIRIS is safe and effective in children younger than 1 month of age.
It is not known if ULTOMIRIS is safe and effective for the treatment of gMG or NMOSD in children.Who should not receive ULTOMIRIS?
Do not receive ULTOMIRIS if you have a serious meningococcal infection when you are starting ULTOMIRIS treatment.Before you receive ULTOMIRIS, tell your healthcare provider about all of your medical conditions, including if you: - have an infection or fever.
- are pregnant or plan to become pregnant. It is not known if ULTOMIRIS will harm your unborn baby.
- Pregnancy Registry: There is a registry for pregnant women who take ULTOMIRIS. The purpose of this registry is to check the health of the pregnant mother and her baby. If you are pregnant or become pregnant while taking ULTOMIRIS, talk to your healthcare provider about how you can join this pregnancy registry or you may contact the registry at 1-833-793-0563 or www.UltomirisPregnancyStudy.com to enroll.
- are breastfeeding or plan to breastfeed. It is not known if ULTOMIRIS passes into your breast milk. You should not breastfeed during treatment and for 8 months after your final dose of ULTOMIRIS.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. ULTOMIRIS and other medicines can affect each other causing side effects.
Know the medicines you take and the vaccines you receive. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.How should I receive ULTOMIRIS? - Your healthcare provider will decide how long you need to receive ULTOMIRIS for your PNH, your aHUS, your gMG, or your NMOSD.
Adults with PNH, aHUS, gMG, or NMOSD when administered intravenously (by vein) - You will be given intravenous ULTOMIRIS infusion by a healthcare provider through a needle placed in a vein
- You will usually receive:
- a starting dose of intravenous ULTOMIRIS infusion by your healthcare provider, and then
- 2 weeks later, you will start to receive an infusion of ULTOMIRIS every 8 weeks.
Children 1 month of age and older with PNH or aHUS when administered intravenously (by vein) Your child will be given intravenous ULTOMIRIS infusion by a healthcare provider through a needle placed in a vein - Your child will usually receive:
- a starting dose of intravenous ULTOMIRIS infusion by your healthcare provider, and then
- your healthcare provider will decide how often your child will receive their intravenous ULTOMIRIS infusion, either every 4 weeks or every 8 weeks, depending on their weight, starting 2 weeks after the starting dose.
If you are changing treatment from SOLIRIS to ULTOMIRIS, you should receive your starting dose of ULTOMIRIS at time of your next scheduled dose of SOLIRIS. - After each administration, you should monitor for infusion-related reactions for at least 1 hour. See "What are the possible side effects of ULTOMIRIS?"
- If you have PNH and you stop receiving ULTOMIRIS, your healthcare provider will need to monitor you closely for at least 16 weeks after you stop ULTOMIRIS. Stopping ULTOMIRIS may cause breakdown of your red blood cells due to PNH.
Symptoms or problems that can happen due to red blood cell breakdown include:
- drop in your red blood cell count
- tiredness
- blood in your urine
- stomach-area (abdomen) pain
- shortness of breath
- blood clots
- trouble swallowing
- erectile dysfunction (ED) in males
- If you have aHUS, your healthcare provider will need to monitor you closely for at least 12 months after stopping treatment for signs of worsening aHUS or problems related to a type of abnormal clotting and breakdown of your red blood cells called thrombotic microangiopathy (TMA).
Symptoms or problems that can happen with TMA may include:
- confusion or loss of consciousness
- seizures
- chest pain (angina)
- difficulty breathing
- blood clots or stroke
If you miss an ULTOMIRIS infusion, call your healthcare provider right away. - ULTOMIRIS can cause serious side effects including: See "What is the most important information I should know about ULTOMIRIS?"
- Infusion-related reactions. Infusion-related reactions may happen during your ULTOMIRIS treatment. Symptoms of an infusion-related reaction with ULTOMIRIS may include lower back pain, stomach (abdominal) pain, muscle spasms, changes in blood pressure, tiredness, feeling faint, shaking chills (rigors), discomfort in your arms or legs, or bad taste. Stop treatment of ULTOMIRIS and tell your healthcare provider right away if you develop these symptoms, or any other symptoms during your ULTOMIRIS infusion that may mean you are having a serious infusion-related reaction, including:
- chest pain
- trouble breathing or shortness of breath
- swelling of your face, tongue, or throat
- feel faint or pass out
The most common side effects of ULTOMIRIS in people treated for PNH are: - upper respiratory tract infection
- headache
The most common side effects of ULTOMIRIS in people treated for aHUS are: - upper respiratory tract infection
- diarrhea
- nausea
- vomiting
- headache
- high blood pressure
- fever
The most common side effects of ULTOMIRIS in people with gMG are: - diarrhea
- upper respiratory tract infections
The most common side effects of ULTOMIRIS in people with NMOSD are: - COVID-19 infection
- headache
- back pain
- urinary tract infection
- joint pain (arthralgia)
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all of the possible side effects of ULTOMIRIS.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of ULTOMIRIS.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about ULTOMIRIS that is written for health professionals.What are the ingredients in ULTOMIRIS? Active ingredient: ravulizumab-cwvz. Inactive ingredients: ULTOMIRIS 100 mg/mL: L-arginine, polysorbate 80 (vegetable origin), sodium phosphate dibasic, sodium phosphate monobasic, sucrose and Water for Injection. ULTOMIRIS 10 mg/mL: polysorbate 80 (vegetable origin), sodium chloride, sodium phosphate dibasic, sodium phosphate monobasic and Water for Injection. Manufactured by Alexion Pharmaceuticals, Inc., 121 Seaport Boulevard, Boston, MA 02210 USA.
U.S. License Number 1743
ULTOMIRIS is a registered trademark of Alexion Pharmaceuticals, Inc.
© 2024 Alexion Pharmaceuticals, Inc.
For more information, go to www.ULTOMIRIS.com or call: 1-888-765-4747. -
ULTOMIRIS increases your chance of getting serious meningococcal infections caused by Neisseria meningitidis bacteria. Meningococcal infections may quickly become life-threatening or cause death if not recognized and treated early.
- PRINCIPAL DISPLAY PANEL - 30 mL Vial Carton
- PRINCIPAL DISPLAY PANEL - 3 mL Vial Carton
- PRINCIPAL DISPLAY PANEL - 11 mL Vial Carton
-
INGREDIENTS AND APPEARANCE
ULTOMIRIS
ravulizumab solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25682-022 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ravulizumab (UNII: C3VX249T6L) (ravulizumab - UNII:C3VX249T6L) ravulizumab 300 mg in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25682-022-01 1 in 1 CARTON 12/21/2018 1 30 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761108 12/21/2018 ULTOMIRIS
ravulizumab solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25682-025 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ravulizumab (UNII: C3VX249T6L) (ravulizumab - UNII:C3VX249T6L) ravulizumab 300 mg in 3 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25682-025-01 1 in 1 CARTON 10/09/2020 1 3 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761108 10/09/2020 ULTOMIRIS
ravulizumab solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25682-028 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ravulizumab (UNII: C3VX249T6L) (ravulizumab - UNII:C3VX249T6L) ravulizumab 1100 mg in 11 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25682-028-01 1 in 1 CARTON 10/09/2020 1 11 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761108 10/09/2020 Labeler - Alexion Pharmaceuticals Inc. (789359510)