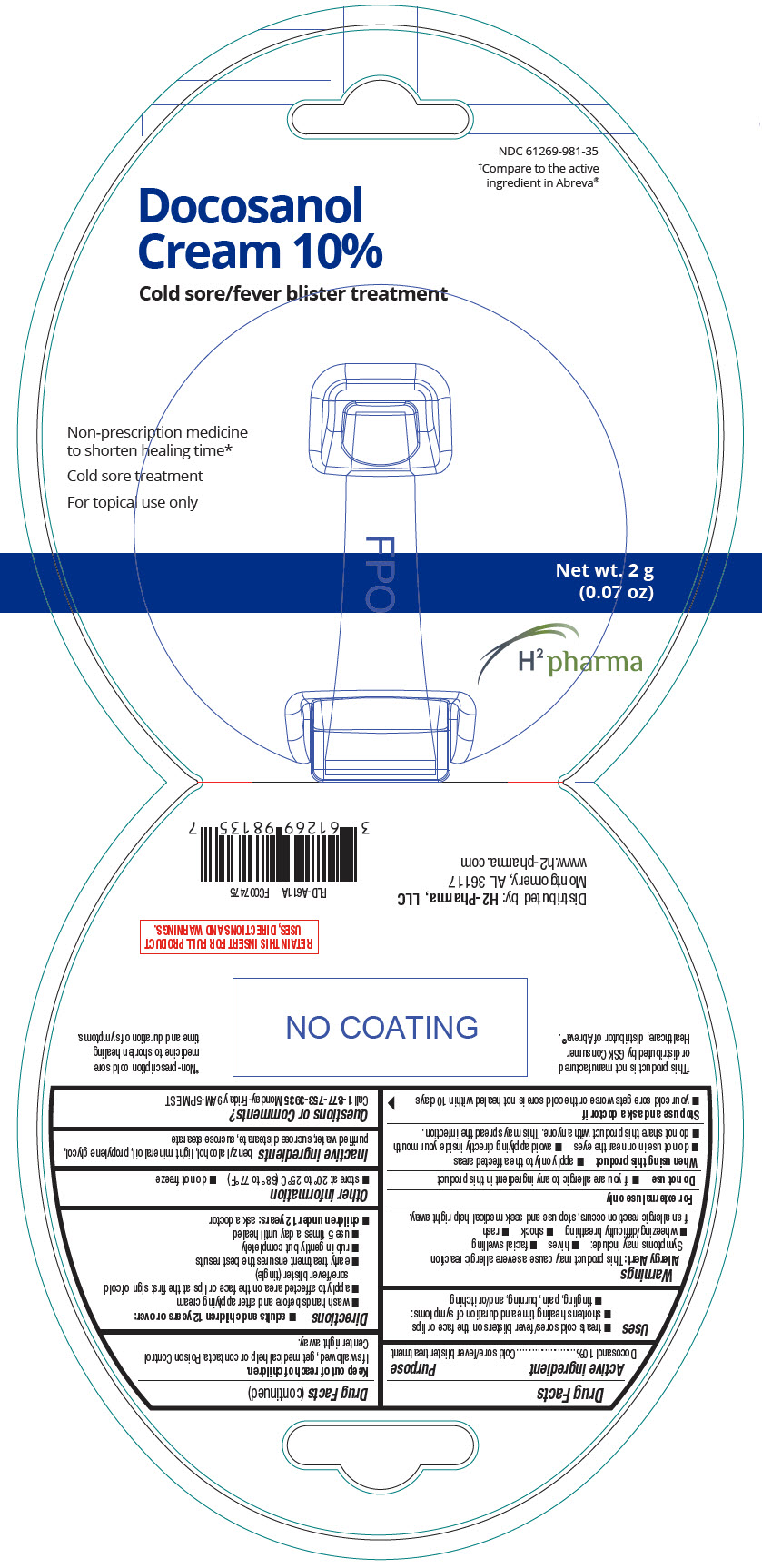
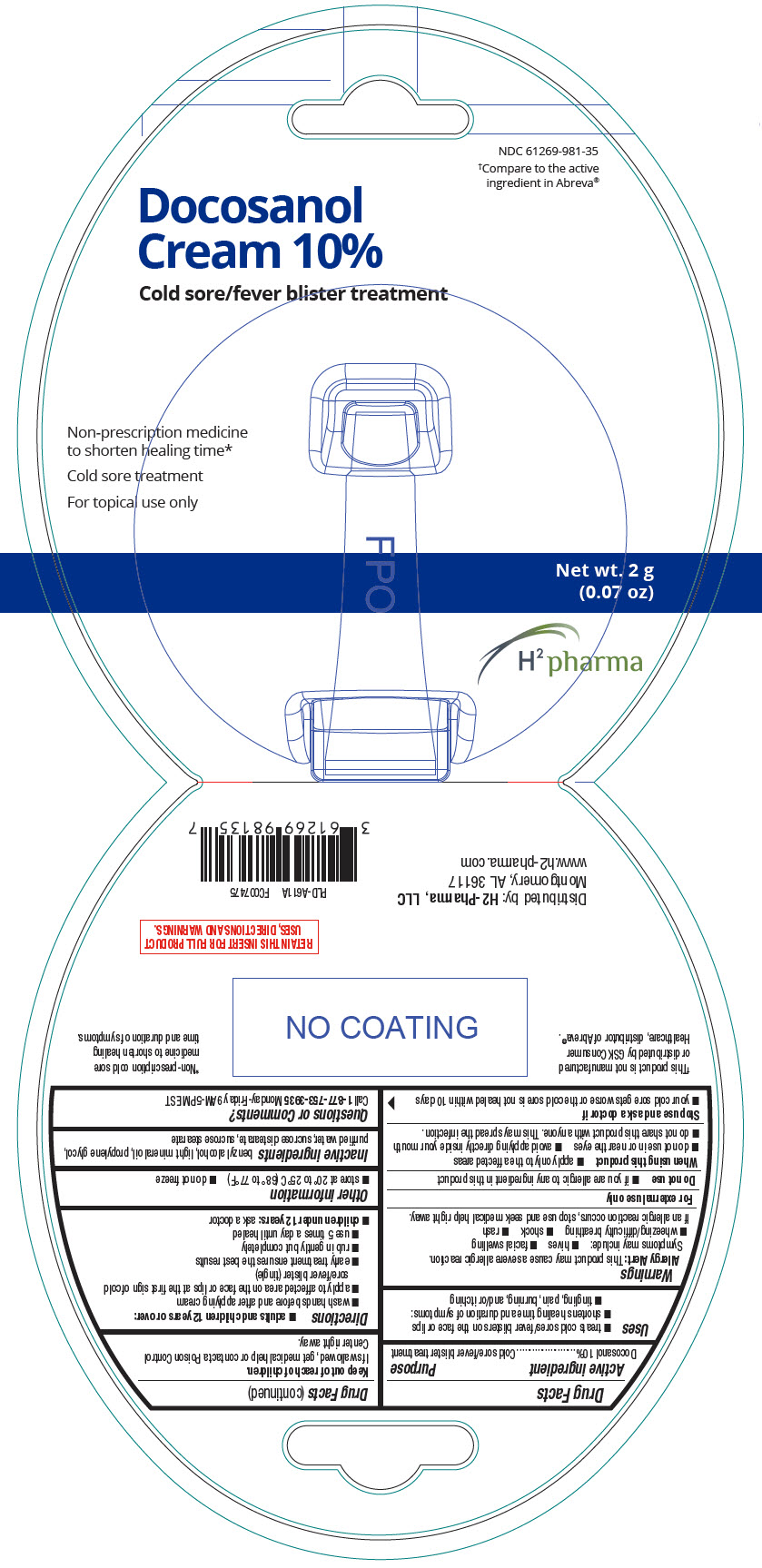
Label: DOCOSANOL cream
- NDC Code(s): 61269-981-35
- Packager: H2-Pharma, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
Allergy Alert
This product may cause a severe allergic reaction. Symptoms may include:
- hives
- facial swelling
- wheezing/difficulty breathing
- shock
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
For external use only
When using this product
- apply only to the affected areas
- do not use in or near the eyes
- avoid applying directly inside your mouth
- do not share this product with anyone. This may spread the infection.
-
Directions
-
adults and children 12 years or over:
- wash hands before and after applying cream
- apply to affected area on the face or lips at the first sign of cold sore/fever blister (tingle)
- early treatment ensures the best results
- rub in gently but completely
- use 5 times a day until healed
- children under 12 years: ask a doctor
-
adults and children 12 years or over:
- Other information
- Inactive ingredients
- Questions or Comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 2 g Tube Package
-
INGREDIENTS AND APPEARANCE
DOCOSANOL
docosanol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61269-981 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCOSANOL (UNII: 9G1OE216XY) (DOCOSANOL - UNII:9G1OE216XY) DOCOSANOL 100 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) LIGHT MINERAL OIL (UNII: N6K5787QVP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SUCROSE DISTEARATE (UNII: 33X4X4B90S) SUCROSE STEARATE (UNII: 274KW0O50M) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61269-981-35 1 in 1 PACKAGE 06/29/2021 1 2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA208754 06/29/2021 Labeler - H2-Pharma, LLC (028473634)