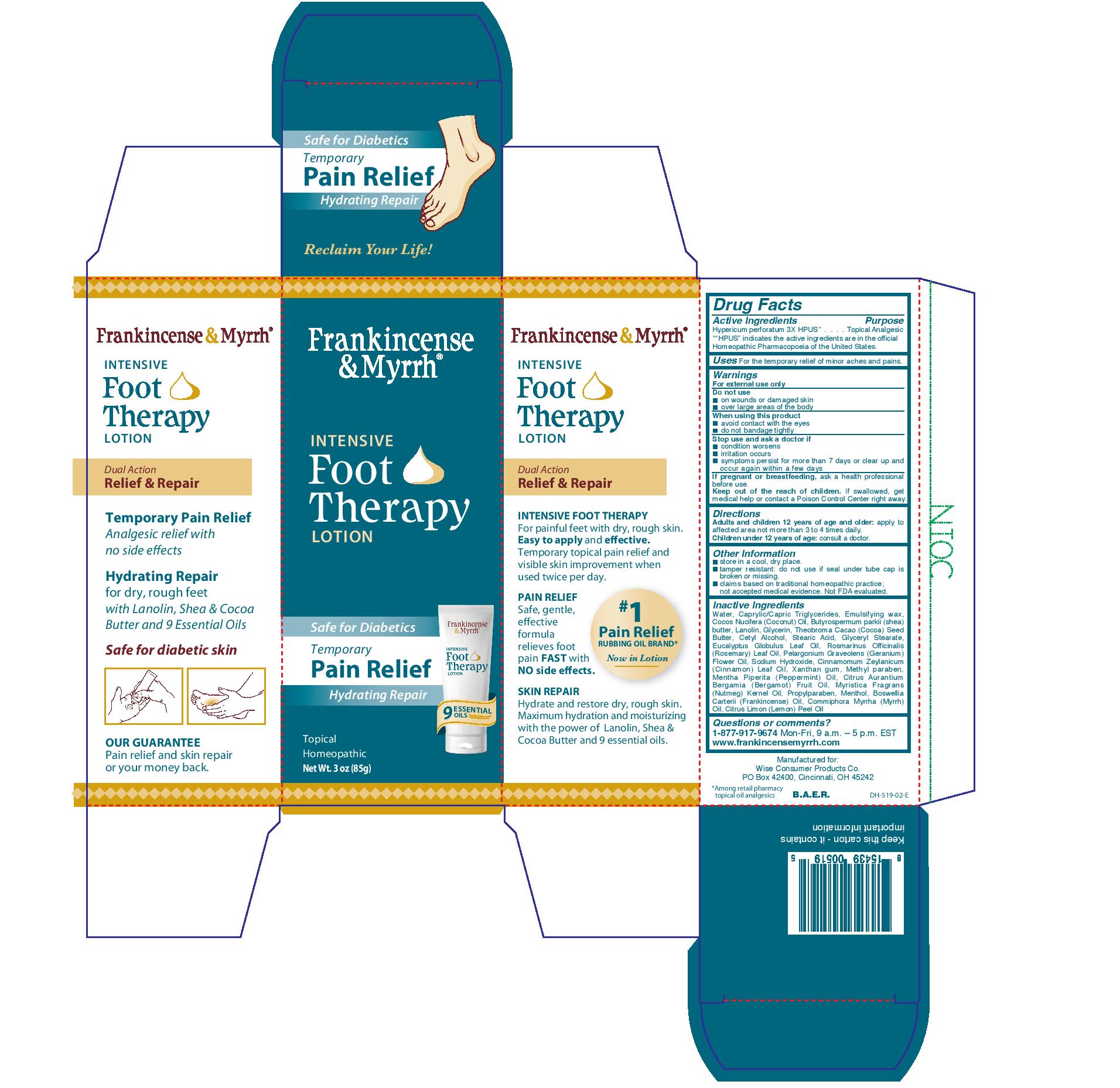
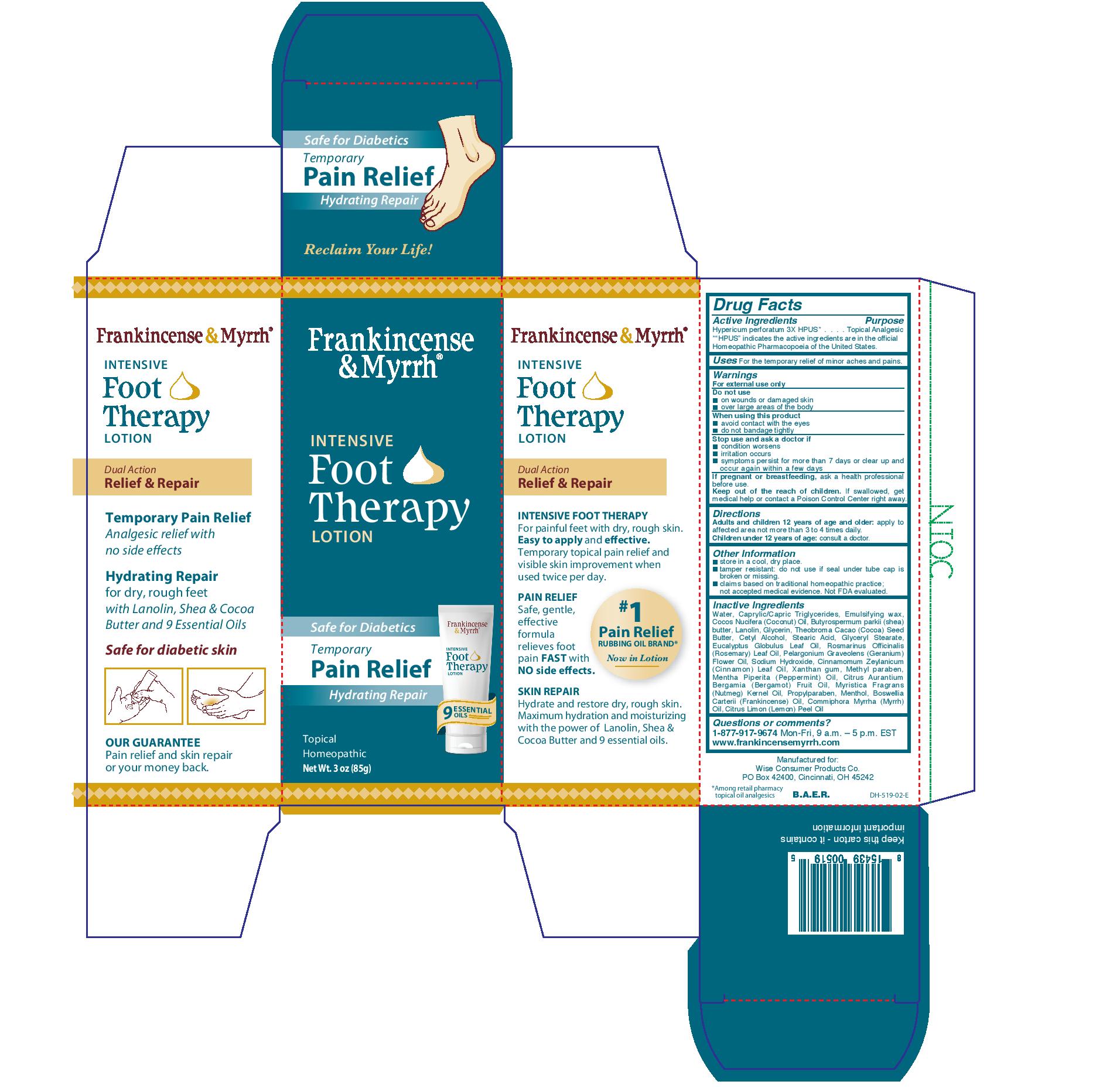
Label: FRANKINCENSE AND MYRRH- intensive foot therapy lotion lotion
- NDC Code(s): 42346-519-85
- Packager: Wise Consumer Products Co.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 27, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Purpose
- Uses
- Warnings
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other Information
-
Inactive Ingredients
Water, caprylic/capric triglycerides, emulsifying wax, coccos nucifera (coconut) oil, butyrospermum parkii (shea) butter, lanolin, glycerin, theobroma cacao (cocoa) seed butter, cetyl alcohol, stearic acid, glyceryl stearate, eucyalyptus globulus leaf oil, romarinus officinalis (rosemary) leaf oil. pelargonium graveolens (geranium) flower oil, sodium hydroxide, cinnamomum zeylanicum (cinnamon) leaf oil, xanthan gum, methyl paraben, mentha piperita (peppermint) oil, citrus aurantium bergamia (bergamot) fruit oil, myristica fragrans (nutmeg) kernel oil, propylparaben, menthol, boswellia carterii (frankincense) oil, commiphora myrrha (myrrh) oil, citrus limon (lemon) peel oil.
- Questions or Comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FRANKINCENSE AND MYRRH
intensive foot therapy lotion lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42346-519 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 3 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength EUCALYPTUS OIL (UNII: 2R04ONI662) CINNAMON LEAF OIL (UNII: S92U8SQ71V) XANTHAN GUM (UNII: TTV12P4NEE) ROSEMARY OIL (UNII: 8LGU7VM393) SHEA BUTTER (UNII: K49155WL9Y) GLYCERIN (UNII: PDC6A3C0OX) CETYL ALCOHOL (UNII: 936JST6JCN) MYRRH OIL (UNII: H74221J5J4) COCONUT OIL (UNII: Q9L0O73W7L) GERANIUM OIL, ALGERIAN TYPE (UNII: 5Q1I94P4WG) METHYLPARABEN (UNII: A2I8C7HI9T) STEARIC ACID (UNII: 4ELV7Z65AP) LANOLIN (UNII: 7EV65EAW6H) SODIUM HYDROXIDE (UNII: 55X04QC32I) MENTHOL (UNII: L7T10EIP3A) WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) COCOA BUTTER (UNII: 512OYT1CRR) PEPPERMINT OIL (UNII: AV092KU4JH) CITRUS AURANTIUM FRUIT OIL (UNII: 59JDQ5VT0T) NUTMEG OIL (UNII: Z1CLM48948) PROPYLPARABEN (UNII: Z8IX2SC1OH) FRANKINCENSE OIL (UNII: 67ZYA5T02K) LEMON OIL (UNII: I9GRO824LL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42346-519-85 1 g in 1 CARTON; Type 0: Not a Combination Product 07/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/01/2020 Labeler - Wise Consumer Products Co. (006459643) Establishment Name Address ID/FEI Business Operations PJ Noyes Co. Inc. 018927392 manufacture(42346-519)