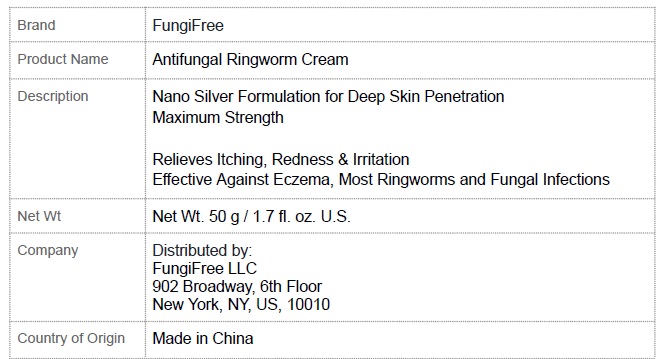
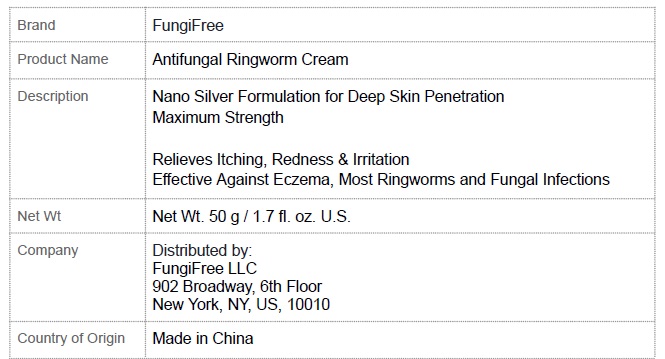
Label: FUNGIFREE ANTIFUNGAL RINGWORM CREAM- menthol,colloidal silver,miconazole cream
- NDC Code(s): 83364-003-01
- Packager: YITONGBADA (SHENZHEN) INTERNATIONAL TRADE CO., LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
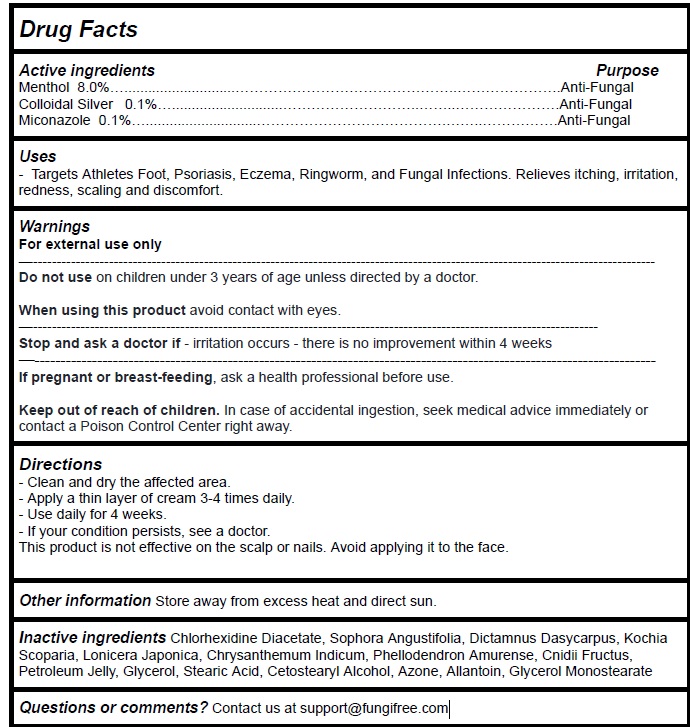
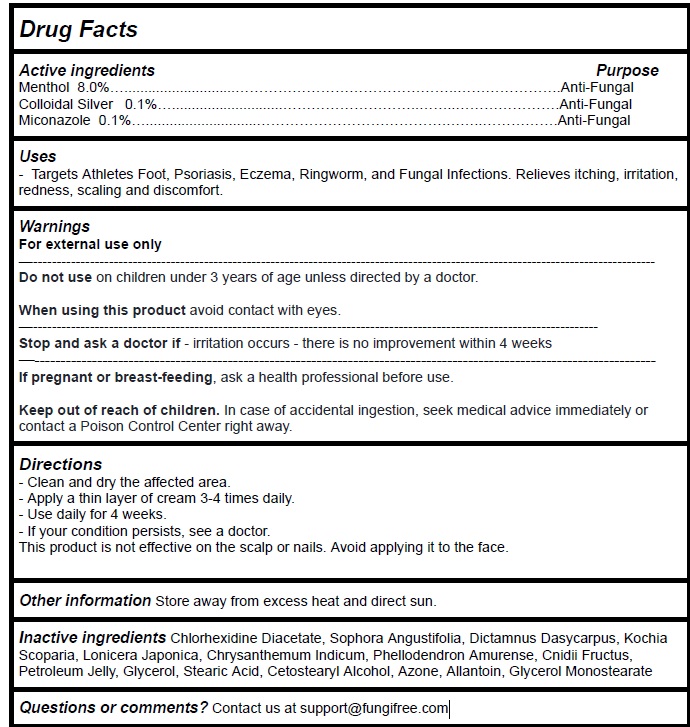
- Active Ingredient Purpose
- PURPOSE
- Uses
-
Warnings
For external use only
Do not use onchildren under 3 years of age unless directed by a doctor.When using this productavoid contact with eyes.
Stop and ask a doctor if- irritation occurs - there is no improvement within 4 weeks
If pregnant or breast-feeding, ask a health professional before use.Keep out of reach of children. In case of accidental ingestion, seek medical advice immediately or contact a Poison Control Center right away.
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- Product label
-
INGREDIENTS AND APPEARANCE
FUNGIFREE ANTIFUNGAL RINGWORM CREAM
menthol,colloidal silver,miconazole creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83364-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 8 g in 100 g SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 0.1 g in 100 g MICONAZOLE (UNII: 7NNO0D7S5M) (MICONAZOLE - UNII:7NNO0D7S5M) MICONAZOLE 0.1 g in 100 g Inactive Ingredients Ingredient Name Strength CHLORHEXIDINE ACETATE (UNII: 5908ZUF22Y) SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) DICTAMNUS DASYCARPUS ROOT (UNII: 6153LEN214) BASSIA SCOPARIA WHOLE (UNII: 240G38P85Z) LONICERA JAPONICA STEM (UNII: NW9XC3TM8R) CHRYSANTHEMUM INDICUM FLOWER (UNII: I6OER6U04L) PHELLODENDRON AMURENSE BARK (UNII: PBG27B754G) CNIDIUM MONNIERI FRUIT (UNII: V1IA3S3CUS) PETROLATUM (UNII: 4T6H12BN9U) GLYCERIN (UNII: PDC6A3C0OX) STEARIC ACID (UNII: 4ELV7Z65AP) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAUROCAPRAM (UNII: 1F3X9DRV9X) ALLANTOIN (UNII: 344S277G0Z) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83364-003-01 1 in 1 BOX 02/25/2024 1 50 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/25/2024 Labeler - YITONGBADA (SHENZHEN) INTERNATIONAL TRADE CO., LTD (725220463)